

Alexandre Lima de Andrade1; Flávia de Rezende Eugênio1; Rosimeri de Oliveira Vasconcelos1; Lilian Bevilacqua2; José Luiz Laus3
DOI: 10.1590/S0004-27492004000100031
ABSTRACT
PURPOSE: To evaluate the u se of equine renal capsule preserved in glycerin to repair lamellar scleral lesions in dogs. METHODS: Twelve healthy mongrel dogs, male and female, weighing 12 kg were used. The study was both clinical and morphological, and performed on the first, third, seventh, 15th, 30th and 60th day after surgery. Temporal canthotomy was performed after standard preoperative and general anesthesia. Conjunctival and scleral square incisions of 0.5x0.5 cm were carried out in a one o'clock position, near the limbus. A fragment of hydrated biological prosthesis, of the same shape, was sutured with 7-0 vicryl® in an interrupted suture. RESULTS: The clinical evaluation showed blepharospasm/photophobia until the 7th day after surgery. Conjunctival edema appeared up to the 5th day after surgery. Mucoid ocular discharge was sustained until the 10th day after surgery. Hyperemia was observed until the end of the evaluation period. There were no signs of graft extrusion in all animals. The anterior and posterior segments did not show clinical signs of inflammation. The optical microscopy morphological evaluation showed an inflammatory exudation with acute aspects in the early and intermediate periods, and inflammatory exudation with chronic aspects in the late periods. There was incorporation of the implant by the recipient's sclera. CONCLUSION: These results suggest that the equine renal preserved capsule could be a useful alternative tissue to repair lamellar corneal lesions in dogs and humans.
Keywords: Sclera; Transplantation heterologous; Dogs; Animal
RESUMO
OBJETIVO: Avaliar o uso da cápsula renal de eqüino preservada em glicerina 98% no reparo de lesões lamelares esclerais em cães. MÉTODOS: Foram utilizados 12 cães, machos e fêmeas, com peso médio de 12kg. Foram realizadas avaliações clínica e morfológica aos 1, 3, 7, 15, 30 e 60 dias de pós-operatório. Após anestesia geral e procedimentos padrões de preparo do campo operatório, foi realizada cantotomia temporal, seguida de incisão conjutival e escleral com área de 0,5x0,5 cm na posição de 1hora, próxima ao limbo. Em seguida, um fragmento de mesma dimensão de cápsula renal de eqüino preservada em glicerina, previamente hidratado em solução salina, foi aplicado ao defeito escleral criado sendo fixado com pontos simples isolados com vicryl 7-0®. RESULTADOS: A avaliação clínica revelou blefaroespasmo/fotofobia até o sétimo dia de pós-operatório. Foi observado edema conjuntival até o quinto dia, acompanhado de secreção ocular mucóide, que persistiu até o décimo dia de pós-operatório. Não foram observados sinais clínicos de rejeição do enxerto em todos os animais, em todos os períodos avaliados. Os segmentos anterior e posterior do bulbo ocular não apresentaram sinais de inflamação. A análise morfológica revelou exsudação inflamatória aguda nos períodos precoces e intermediários da avaliação e inflamação crônica nos períodos tardios da observação. Houve incorporação do enxerto ao leito receptor. CONCLUSÃO: Os resultados sugerem que a cápsula renal de eqüino preservada pode ser mais uma alternativa de membrana biológica para o reparo de lesões esclerais lamelares em cães e no homem.
Descritores: Esclera; Transplante heterólogo; Cães; Animal
SHORT REPORT
Repair of lamellar scleral lesions in dogs with preserved equine renal capsule - Short report
Reparação de lesões lamelares esclerais em cães com cápsula renal de eqüino preservada - Relato curto
Alexandre Lima de AndradeI; Flávia de Rezende EugênioII; Rosimeri de Oliveira VasconcelosII; Lilian BevilacquaIII; José Luiz LausIV
IProfessor Assistente Doutor do Curso de Medicina Veterinária da Faculdade de Odontologia de Araçatuba, da Universidade Estadual Paulista - UNESP - Campus de Araçatuba
IIProfessora Assistente Doutora do Curso de Medicina Veterinária da Faculdade de Odontologia de Araçatuba da Universidade Estadual Paulista - UNESP - Campus de Araçatuba
IIIMédica Veterinária Residente do Hospital Veterinário do Curso de Medicina Veterinária da Faculdade de Odontologia de Araçatuba da Universidade Estadual Paulista - UNESP - Campus de Araçatuba
IVProfessor Titular da Faculdade de Ciências Agrárias e Veterinárias da Universidade Estadual Paulista - UNESP - Campus de Jaboticabal
ABSTRACT
PURPOSE: To evaluate the u se of equine renal capsule preserved in glycerin to repair lamellar scleral lesions in dogs.
METHODS: Twelve healthy mongrel dogs, male and female, weighing 12 kg were used. The study was both clinical and morphological, and performed on the first, third, seventh, 15th, 30th and 60th day after surgery. Temporal canthotomy was performed after standard preoperative and general anesthesia. Conjunctival and scleral square incisions of 0.5x0.5 cm were carried out in a one o'clock position, near the limbus. A fragment of hydrated biological prosthesis, of the same shape, was sutured with 7-0 vicryl® in an interrupted suture.
RESULTS: The clinical evaluation showed blepharospasm/photophobia until the 7th day after surgery. Conjunctival edema appeared up to the 5th day after surgery. Mucoid ocular discharge was sustained until the 10th day after surgery. Hyperemia was observed until the end of the evaluation period. There were no signs of graft extrusion in all animals. The anterior and posterior segments did not show clinical signs of inflammation. The optical microscopy morphological evaluation showed an inflammatory exudation with acute aspects in the early and intermediate periods, and inflammatory exudation with chronic aspects in the late periods. There was incorporation of the implant by the recipient's sclera.
CONCLUSION: These results suggest that the equine renal preserved capsule could be a useful alternative tissue to repair lamellar corneal lesions in dogs and humans.
Keywords: Sclera/lesions; Transplantation heterologous; Dogs; Animal
RESUMO
OBJETIVO: Avaliar o uso da cápsula renal de eqüino preservada em glicerina 98% no reparo de lesões lamelares esclerais em cães.
MÉTODOS: Foram utilizados 12 cães, machos e fêmeas, com peso médio de 12kg. Foram realizadas avaliações clínica e morfológica aos 1, 3, 7, 15, 30 e 60 dias de pós-operatório. Após anestesia geral e procedimentos padrões de preparo do campo operatório, foi realizada cantotomia temporal, seguida de incisão conjutival e escleral com área de 0,5x0,5 cm na posição de 1hora, próxima ao limbo. Em seguida, um fragmento de mesma dimensão de cápsula renal de eqüino preservada em glicerina, previamente hidratado em solução salina, foi aplicado ao defeito escleral criado sendo fixado com pontos simples isolados com vicryl 7-0®.
RESULTADOS: A avaliação clínica revelou blefaroespasmo/fotofobia até o sétimo dia de pós-operatório. Foi observado edema conjuntival até o quinto dia, acompanhado de secreção ocular mucóide, que persistiu até o décimo dia de pós-operatório. Não foram observados sinais clínicos de rejeição do enxerto em todos os animais, em todos os períodos avaliados. Os segmentos anterior e posterior do bulbo ocular não apresentaram sinais de inflamação. A análise morfológica revelou exsudação inflamatória aguda nos períodos precoces e intermediários da avaliação e inflamação crônica nos períodos tardios da observação. Houve incorporação do enxerto ao leito receptor.
CONCLUSÃO: Os resultados sugerem que a cápsula renal de eqüino preservada pode ser mais uma alternativa de membrana biológica para o reparo de lesões esclerais lamelares em cães e no homem.
Descritores: Esclera/lesões; Transplante heterólogo; Cães; Animal
INTRODUCTION
A number of specific inflammatory responses affects the episclera and sclera of dogs. The common association of episcleritis or scleritis with rheumatoid arthritis in humans, however, has not been established in dogs(1). Inflammatory diseases of the sclera can be divided into nonnecrotizing granulomatous scleritis and necrotizing granulomatous scleritis. In advanced scleritis, there may be diffuse corneal stromal infiltration, as well as secondary retinal involvement. The scleral lesions may, or may not, be associated with systemic collagen diseases(2). In these cases, they may progress to complete perforation or require specific therapy to prevent perforation.
Preserved biological membranes have been used in human and veterinary ophthalmology with good results for corneal and scleral tectonic repair(3-6). Surgical techniques employed for scleromalacia include homologous grafts of sclera(7-8), fascia lata graft(9-12); aorta graft(13) and preserved heterologos pericardium(14).
Despite the numerous surgical techniques that have been described, there are few reports on xenogeneic grafts for tectonic scleral repair. Thus, the purpose of this study was to evaluate the preserved equine renal capsule for repair of the experimental lamellar scleral lesions in dogs.
METHODS
Experimental design
Twelve adult, 12-kg, mongrel dogs, divided into six groups (n=2), of one male and one female, were studied. In order to study the postoperative course, six groups of two animals were named: G1, G2, G3, G4, G5 and G6. These groups were analyzed via ophthalmic examinations with the slit-lamp biomicroscope, before and after the lamellar scleratoplasties, and placement of an equine renal capsule graft.
Biological prosthesis
The equine renal capsule was preserved in 98% glycerine(3-4,14). The biological prosthesis was harvested from horses that were euthanized due to orthopedic fractures but otherwise healthy.
Surgical technique
The surgical technique was performed on 12 clinically healthy dogs (each dog had one eye surgically treated). The animals were anesthetized with halothane. After a lateral canthotomy, the surgery was performed with the aid of an operating microscope 40X magnification (Microscope MC-M9). Conjunctival and scleral square incisions of 0.5x0.5 cm were carried out in a 1 o'clock position, near the limbus. For superficial sclerectomies, a trephine was used to create a half-thickness defect in the sclera. A fragment of hydrated biological prosthesis was rinsed and stored in sterile 0.9% saline, and cut with the same dimensions as the graft bed. Then, the prosthesis was fixed in its recipient bed using an interrupted pattern, 8-0 vicryl® suture. After surgery, the dogs were treated with topically applied chlorphenicol ointment, twice a day for 10 days, in order to prevent bacterial infection.
Clinical and morphological evaluation
The clinical evaluations were performed at 24-h intervals. The ocular signs evaluated were blepharospasm/photophobia, ocular discharge, conjunctival edema and hyperemia(3). These evaluations were performed by two observers.
The light microscopy study examined periods of one, three, seven, 15, 30 and 60 days after surgery. Six-micrometer section samples were stained with hematoxylin-eosin (H&E). The scleras, which received the grafts, were then analyzed.
RESULTS
Clinical evaluation
All scleras had marked conjunctival edema in the early postoperative period, which then decreased along 3 days after surgery. Blepharospasm/photophobia and ocular discharge were marked in the early period. Hyperemia was very significant in the early postoperative period and decreased in the late period.
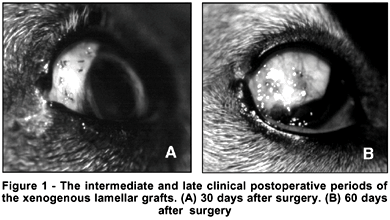
In this study neither pigmentation of the graft bed, nor extrusion of the graft, as well as signs of inflammation of the posterior and anterior segments were observed. Figure 1 illustrates the clinical aspects on the 30th and 60th day after surgery.
Histopathological evaluation
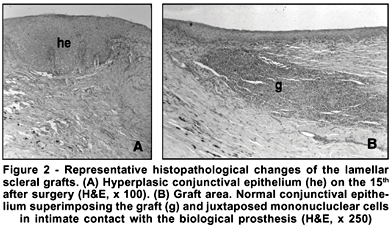
All dogs followed a similar pattern during the healing process. The main histopathologic changes were those at the junction of the graft tissue and beneath the graft bed. Polymorphonuclear leukocytes were observed in the beginning of the inflammatory process until the 15th after surgery; however, by day 15 the number of mononuclear cells had increased and continued to do so until day 60. On day 7, the conjunctival epithelium was hyperplasic on the graft and inflammation infiltrate plasma cells and lymphocytes in graft bed were observed. The basal cell proliferation started 24 h after surgery. These cells migrated juxtaposed in intimate contact with the biological prosthesis, especially superimposing the whole graft on the third day. The number of globet cells had increased on day 15. On day 60, the biological prosthesis was atrophic and degenerated; however, there were not alterations of the conjunctival epithelium and sclera beneath the graft. The results of the microscopic study are summarized in figure 2.
DISCUSSION
Many methods have been described for repair of deep corneal ulcers and perforations, and yet, with the exception of penetrating keratoplasties with autogenous grafts, corneal opacification may be the final result(3). In humans, biological prosthesis can be performed by reconstructing the anterior ocular segment(5,15).
Our results showed that photophobia/blepharospasm/epiphora were significant in the early period after surgery(3), and may be due to the scleral edema stimulating the scleral nerves and the sutures stimulating the conjunctival eyelid. Sutures were not removed to avoid additional maneuvres that could possibly interfere in the surgical site.
Possible complications associated with this procedure include postoperative infection and progression of the autolytic process by leukocyte collagenase or bacterial protease which may be present(16-17). However, they were not observed. Solutions for preservation of corneal tissue in human medicine, have been described(18-19). Nevertheless, the storage procedure increased the cost. Alternatively, the use of 98% glycerine for biological prosthesis preservation for up to 30 days has been described(3-4). This solution was an excellent fluid for preservation of our grafts and was rather inexpensive.
In the few reports about scleratoplasty, the authors did not study the cicatricial reparation kinectics using light microscopy(14). The first reported a study using preserved equine renal capsule to repair lamellar scleral lesions in dogs. Although the histopathological changes in graft areas has shown inflammation, necrosis and atrophy areas and increased number of globet cells, the graft was integrated into the canine sclera with no detectable clinical and histologically rejection.
CONCLUSIONS
In conclusion, renal capsule grafting may be appropriate for treatment of scleral lesions that may progress to complete perforation or to prevent it. This biological prosthesis was tolerated on clinical and histopathological evaluation. Based on this, the preserved equine renal capsule might represent a reasonable alternative to sclera in such procedures in humans as observed in other studies.
REFERENCES
1. Fischer CA. Inflammatory diseases of the sclera and episclera. In: Peiffer RL Jr, editor. Comparative ophthalmic pathology. Springfield: Charles C. Thomas; 1983. p.272-88.
2. Gelatti KN, editor. Veterinary ophthalmology. 3rd ed. Philadelphia: Lippincott Williams & Willkins; 1999. 1544p.
3. Andrade AL, Laus JL, Figueiredo F, Batista CM. The use of preserved equine renal capsule to repair lamellar corneal lesions in normal dogs. Vet Ophthalmol 1999; 2:79-82.
4. Barros PSM, Garcia JA, Laus JL, Ferreira A, Salles Gomes TL. Preserved equine amniotic membrane used in the repair of the cornea of the dog. Invest Ophthalmol Vis Sci 1995;36:982-85.
5. Rodríguez-Ares MT; Touriño R, Capeans C, Sanches-Salorio M. Repair of scleral perforation with preserved scleral and amniotic membrane in Marfan's syndrome. Ophthalmic Surg Lasers 1999;30:485-7.
6. Nguyen QD, Foster CS. Scleral patch graft in the management of necrotizing scleritis. Int Ophthalmol Clin 1999;39:109-31.
7. Obear MF, Winter FC. Technique of overlay scleral homograft. Arch Ophtalmol 1964;71:837-8.
8. Sevel D, Abramson A. Necrogranulomatous scleritis treated by an onlay scleral graft. Br J Ophthalmol 1972;56:791-9.
9. Bick MW. Surgical treatment of scleromalacia perforans. Ama Arch Ophtalmol 1959;61:907-17.
10. Taffet S, Carter GZ. The use of a fascia lata graft in the treatment of scleromalacia perforans. Am J Ophthalmol 1961;52:693-6.
11. Torchia RT, Dunn RE; Peasl PJ. Fascia lata grafting in scleromalacia perforans with lamellar corneal-scleral dissection. Am J Ophthalmol 1968;66:705-9.
12. Zer I, Machtey I, Kurz O. Combined treatment of scleromalacia perforans in rheumatoid arthritis with penicillamine and plastic surgery. Ophthalmologica 1973;166:293-300.
13. Merz, EH. Scleral reinforcement with aortic tissue. Am J Ophthalmol 1964; 57:766-70.
14. Barros PSM, Laus JL, Morales A, Ferreira AL, Safatle AMV. Reparação experimental de lesões lamelares da esclera de cães com pericárdio xenólogo conservado. Arq Bras Oftalmol 1996;59:462-6.
15. Schein OD. The use of processed pericardial tissue in anterior ocular segment reconstruction. Am J Ophthalmol 1998;125:549-52.
16. Hacker DV. Frozen corneal grafts in dogs and cats: a report on 19 cases. J Am Anim Hosp Assoc 1991;27:387-98.
17. DeBacker CM, Dutton JJ, Proia AD, Stone T, Holck DE. A comparative study of bovine pericardium (periguard) and homologous sclera as lower eyelid spacer graft analogs in New Zealand white rabbits. Ophthal Plast Reconstr Surg 2000; 16:156-61.
18. MacCarey BE, Kaufman HE. Improved corneal storage. Invest Ophthalmol 1974;13:165-73.
19. Yau CW, Kaufman HE. A medium-term corneal preserving medium (K-Sol). Arch Ophthalmol 1986;104:598-601.
 Correspondence to
Correspondence to
Rua Clóvis Pestana, 793
Araçatuba (SP) CEP 16050-680
E-mail: [email protected]
Recebido para publicação em 10.07.2002
Versão revisada recebida em 06.01.2003
Aprovação em 07.04.2003