

Natasha Ferreira Santos da Cruz1; Carolina Ando Matsuno1; Sérgio Ferreira Santos da Cruz2; Aline Pimentel de Miranda1; José Vital Filho1
DOI: 10.5935/0004-2749.20180033
ABSTRACT
Undifferentiated pleomorphic sarcoma (UPS) is an extremely rare tumor that occurs in the head and neck region. Here, we report a unique case of a primary undifferentiated pleomorphic sarcoma in the orbital region. A 35-year-old woman presented with a progressive proptosis and periocular edema for 1 year. She had no previous history of surgery, skin malignancy, or radiation. Imaging tests showed an extraconal mass, not involving the muscles. The tumor was surgically removed and adjuvant radiotherapy was required after histological examination, which showed an undifferentiated pleomorphic sarcoma of the orbit. There was no recurrence after 1 year of follow-up. Though rare, undifferentiated pleomorphic sarcoma should be included in the differential diagnoses of orbital tumors.
Keywords: Histiocytoma, malignant fibrous; Sarcoma; Exophthalmos; Orbital neoplasms; Humans; Case reports
RESUMO
O sarcoma pleomórfico indiferenciado (SPI) é um tumor extremamente raro na região da cabeça e pescoço. Relatamos um caso de um sarcoma pleomórfico indiferenciado primário na região orbital. Uma mulher de 35 anos apresentou proptose progressiva e edema periocular há um ano. Ela não tinha histórico prévio de cirurgia, malignidade da pele ou radiação. Exames de imagem mostraram uma massa extraconal, poupando os músculos. O tumor foi removido cirurgicamente e foi necessária radioterapia adjuvante após o resultado histopatológico. O exame histológico demonstrou um sarcoma pleomórfico indiferenciado da órbita. Não houve recidiva após 1 ano de seguimento. Apesar de raro, o sarcoma pleomórfico indiferenciado deve ser incluído no diagnostico diferencial de qualquer tumor originado na órbita.
Descritores: Histiocitoma fibroso maligno; Sarcoma; Exoftalmia; Neoplasias orbitárias; Humanos; Relatos de casos
INTRODUCTION
Undifferentiated pleomorphic sarcoma (UPS), formerly known as malignant fibrous histiocytoma, has been included in the World Health Organization's classification of soft tissue tumors since 2002. The term "malignant fibrous histiocytoma" was previously used as a collective term for soft tissue malignancies that have no distinct components, and included most soft tissue sarcomas(1). With reclassification, this term has become a diagnosis of exclusion indicating failure to show any specific differentiation using currently available ancillary techniques, and exclusion of epithelial, melanotic, and lymphoid differentiation. These neoplasms account for 5%-10% of malignant sarcomas and occur more commonly in the extremities(2). Tumors arising in the head and neck are extremely rare and involve approximately 3% of all undifferentiated pleomorphic sarcomas(3). UPS most frequently affects older Caucasian males, especially in damaged skin and is typically a large, deep-seated tumor, which shows progressive and often rapid enlargement. Only those that grow very rapidly tend to be painful. Approximately 5% of patients have metastases at presentation, most often to the lung(1). This case report describes a patient with an extremely rare growing and primary, undifferentiated, pleomorphic sarcoma originating from the orbit over a period of 1 year.
CASE REPORT
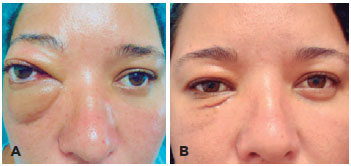
A 35-year-old woman was admitted to the hospital with progressive proptosis and edema of the right eye for 1 year (Figure 1 A). Visual acuity (VA) was 20/20 in both eyes, and other ophthalmic examination results were within normal ranges. The patient denied any personal or familial antecedents of interest. Laboratory tests were performed, including analyses of thyroid hormones, with normal results.
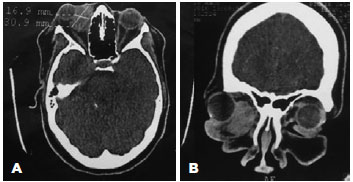
Computed tomography and magnetic resonance imaging of the orbit revealed an extraconal lesion with contrast enhancement and an expansive unencapsulated process located in the inferomedial orbital compartment, with imprecise limits, without bone involvement, and without infiltration of the globe or orbital muscles (Figure 2). Further systemic investigation did not reveal additional tumor foci and/or metastases.
The tumor was excised using medial inferior orbitotomy, without muscle deinsertion (Figure 3 A). After the procedure, the VA remained the same, but the patient exhibited diplopia in the primary position, which improved in 2 weeks (Figure 1 B).
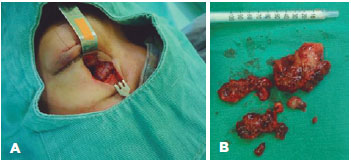
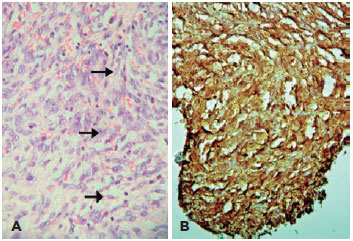
Anatomopathological analysis revealed three nodules measuring 4.0 × 3.7 cm, 3.5 × 1.0 cm, 3.5 × 0.7 cm (Figure 3 B), which were characterized by dense proliferation of large and fusiform cells, with disorderly arranged marked polymorphism and atypia nuclei, interspersed by numerous blood capillaries (Figure 4A). Immunohistochemistry tests were positive for CD34 (Figure 4 B) and vimentin, inconclusive for S-100 protein, and negative for desmin (muscle), SMA, melan-A, HMB45, Bcl-2, cytokeratin, CD10, CD20, CD31, CD68, and XIIIa factor. Ki-67/MIB-1 antigen, a prognostic aggressiveness marker, was 15%. The final diagnosis was undifferentiated pleomorphic orbital sarcoma.
The tumor did not exhibit bone erosion, and the level of the MIB-1 tumor aggressiveness prognostic marker was low, so subsequent surgery was not performed due to possible morbidity and to reduce functional and cosmetic costs to the young patient. However, the tumor limits were not free after a broad excision, and the patient was referred for radiotherapy to complete the treatment. She was treated with a total dose of 54 Gy, which resulted in remission. After 1 year of follow-up with imaging tests to screen for tumor relapse and metastasis, no sign of the disease was found.
DISCUSSION
Undifferentiated sarcoma is a malignant tumor of mesenchymal origin. Although little is known about its etiology, a subset of pleomorphic sarcomas (<2%-3%) arise at the site of prior radiation therapy and, in rare cases, arise at the site of chronic ulceration or scarring(1).
Histologically, the tumor does not exhibit a specific morphology. It is comprised of marked cytological and nuclear pleomorphism, often with bizarre giant tumor cells, mixed with spindle cells, and often with rounded histiocyte-like cells (which may have a foamy cytoplasm) in varying proportions(1).
The role of immunohistochemistry in the diagnosis of UPS has traditionally been an ancillary one, primarily to exclude other pleomorphic tumors. Thus, the diagnosis continues to presuppose thorough sampling and evaluation of hematoxylin-eosin-stained sections. Despite the limited diagnostic applications of immunohistochemistry, except for excluding other lesions, there is sufficient evidence that these tumors do not display features of monocytes or macrophages but, instead, display features of fibroblasts and/or myofibroblasts(4).
Sarcomatoid carcinoma is distinguished from undifferentiated pleomorphic sarcoma by positive staining for cytokeratin. However, positive immunostaining results for S-100, melan-A, and HMB-45 are valuable to differentiate this tumor from melanomas. CD10 and CD20 immunostaining assists in differentiating pleomorphic sarcomas from lymphomas. In addition, the following immunostaining results assist in differentiating pleomorphic sarcomas from similar tumors: CD31 from angiosarcomas, CD68 from histiocytic sarcomas, XIIIa factor from dermatofibromas, and Bcl-2 from solitary fibrous tumors. Judicious use of additional immunohistochemistry can identify the origin of tumors to eliminate pleomorphic leiomyosarcomas (SMA, desmin, and h-caldesmon), pleomorphic rhabdomyosarcomas (desmin), and pleomorphic liposarcomas (S-100 and SMA). Ki-67 is a nuclear antigen present in all non-G0 phases of the cell cycle, and is therefore a useful marker to evaluate neoplastic cell proliferation. In general, the Ki-67 indices correlate with the histological grades(5).
In the present case, some tumor cells were positive for CD34, a mesenchymal differentiation marker, and vimentin, an intermediate filament marker with poor specificity that is present in most mesenchymal cells, sarcomas, melanomas, and variable in lymphomas and carcinomas. However, they were negative for the remaining immunohistochemical markers5. We therefore excluded these possibilities and diagnosed the patient with an undifferentiated pleomorphic sarcoma.
Loco-regional and systemic extension studies must always be performed. In radiological imaging studies, these tumors are usually circumscribed. Infiltration of the extraocular muscles, which can also produce bone erosion, has been previously described(6).
The present patient did not have thyroid disease, and imaging revealed an extraconal tumor that did not involve the muscles. The differential diagnosis could also include other causes of differentiated orbitary masses, such as cavernous hemangioma, tumors derived from nerve sheaths, and other vascular neoplasia such as hemangiopericytomas(6).
Wide excision followed by irradiation is the treatment of choice(1,2,7,8). This type of surgical resection is dependent on various parameters, including tumor location, tumor size, tumor depth, invasion of adjacent structures, a requirement for reconstruction, and the patient's general condition(9). Previous studies reported that 85% of the patients treated with partial resection and subsequent radiotherapy had favorable prognoses after 5 years(10). In addition, adjuvant chemotherapy may be considered in select cases (e.g., young patients)(2).
UPS has complex karyotypes, with extensive intratumoral heterogeneity and no specific structural or numerical abnormalities that are useful for identification(2).
Local recurrence is frequent, but loco-regional remote metastasis is rare (approximately 5%). In cases of remote extension, the treatment of choice is surgery with adjuvant chemotherapy and subsequent radiotherapy(11). Regular follow-up of patients is essential for controlling possible local relapses or remote metastasis(10). The present patient did not exhibit tumor relapse or metastasis after a follow-up period of 1 year.
REFERENCES
1. Fletcher CD, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone [Internet]. Lyon: IARC Press; 2002. (World Health Organization Classification of Tumours). [cited 2016 Jul 21]. Available from: https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb5/BB5.pdf
2. Guillou L. Pleomorphic sarcomas: subclassification, myogenic differentiation and prognosis. Diagn Histopathol. 2008;14(11):527-37.
3. Barnes L, Eveson JW, Reichart P, e Sidransky D, editors. Pathology and genetics of head and neck tumours [Internet]. Lyon: IARC Press; 2004. (World Health Organization Classification of Tumours). [cited 2016 Jul 21]. Available from: https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf
4. Goldblum JR. An approach to pleomorphic sarcomas: can we subclassify, and does it matter? Mod Pathol. 2014;27 Suppl 1:S39-46.
5. Dabbes DJ. Diagnostic Immunohistochemistry: theranostic and genomic applications. 4th ed. Philadelphia (PA): Elsevier/Saunders; 2014.
6. Jacom-Hood J, Moseley IF. Orbital fibrous histiocytoma: computed tomography in 10 cases and a review of radiological findings. Clin Radiol. 1991;43(2):117-20.
7. Müller-Richter UD, Kohlhof JK, Reichert TE, Roldán JC. Undifferentiated pleomorphic sarcoma of the orbital region. Br J Oral Maxillofac Surg. 2008;46(4):325-7.
8. Muñoz Gallego A, Mencia Gutiérrez E, Cámara Jurado M, Gallego Gallego MS, Gutiérrez Dias E. [Undifferentiated high-grade pleomorphic sarcoma of the orbit: a case report]. Arch Soc Esp Oftalmol. 2014;89(10):425-7.
9. Choi W, Hwang JH, Kim GE, Yoon KC. Aggressively progressing primary undifferentiated pleomorphic sarcoma in the eyelid: A case report and review of the literature. Medicine. 2017;96(18):e6616.
10. Delaney TF, Kepka L, Goldberg SI, Hornicek FJ, Gebhardt MC, Yoon SS, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007; 67(5):1460-9.
11. Vuity D, Bodgan S, Csurgay K, Sapi Z, Nemeth Z. Malignant fibrous histiocytoma/undifferenciated high-grade pleomorphic sarcoma of the maxillary sinus: report of a case and review of literature. Pathol Oncol Res. 2013;19(4):605-9.
Submitted for publication:
June 1, 2017.
Accepted for publication:
December 19, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.