

Mehmet Kaygisiz1; Ufuk Elgin2; Kemal Tekin3; Emine Sen2; Pelin Yilmazbas2
DOI: 10.5935/0004-2749.20180025
ABSTRACT
Purpose: To compare the anterior segment parameters of patients with pseudoexfoliation syndrome, patients with pseudoexfoliation glaucoma, and normal subjects.
Methods: This prospective, controlled, comparative study included 150 eyes of 150 patients. The patients were divided into the pseudoexfoliation syndrome group, the pseudoexfoliation glaucoma group, and the control group (50 patients in each group). Axial length, central corneal thickness, aqueous depth, anterior chamber depth, lens thickness, K1 and K2 keratometry values, and white to white distance measurements were obtained by optical biometry and compared between the groups.
Results: The mean ages of the pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and control patients were 62.18 ± 6.21, 61.80 ± 6.62, and 59.40 ± 6.89 years, respectively. There were no statistically significant differences between the groups in mean age or sex ratio (p>0.05). Mean central corneal thickness was statistically significantly greater, mean aqueous depth and anterior chamber depth were statistically significantly greater, and mean lens thickness was statistically significantly less in the control group than in the pseudoexfoliation syndrome and pseudoexfoliation glaucoma groups (p<0.05). Pairwise comparisons of the pseudoexfoliation syndrome group and the pseudoexfoliation glaucoma group revealed that there were no significant differences between these two groups in central corneal thickness, aqueous depth, anterior chamber depth, and lens thickness (p>0.017).
Conclusions: Patients with pseudoexfoliation glaucoma and pseudoexfoliation syndrome had greater lens thickness, shallower aqueous depth and anterior chamber depth, and less central corneal thickness than normal subjects. None of the anterior segment parameters differed between patients with pseudoexfoliation syndrome and patients with pseudoexfoliation glaucoma.
Keywords: Anterior eye segment; Cornea; Lens; Glaucoma; Exfoliation syndrome
RESUMO
Objetivos: Comparar os parâmetros do segmento anterior de casos de síndrome de pseudo-esfoliação, de glaucoma pseudo-esfoliação e de indivíduos normais.
Métodos: O presente estudo prospectivo comparativo controlado incluiu 150 olhos de 150 pacientes. Os pacientes foram divididos em três grupos: grupo síndrome de pseudo-esfoliação, grupo glaucoma pseudo-esfoliação e grupo controle (50 em cada grupo). O comprimento axial, a espessura corneana central, a profundidade aquosa, a profundidade da câmara anterior, a espessura da lente, os valores de ceratometria K1 e K2 e as medidas branco a branco, obtidas por biometria óptica, foram comparados entre os grupos.
Resultados: As idades médias dos indivíduos do grupo síndrome de pseudo-esfoliação, glaucoma pseudo-esfoliação e controle foram 62,18 ± 6,21, 61,80 ± 6,62 e 59,40 ± 6,89 anos, respectivamente. Entre os grupos, não houve diferenças estatisticamente significativas quanto às idades e ao gênero dos pacientes (p>0,05, para todos). A espessura da córnea central média foi significativamente mais espessa, a profundidade média aquosa e a profundidade da câmara anterior foram significativamente mais profundas e a espessura média da lente foi significativamente mais fina no grupo controle do que nos grupos síndrome de pseudo-esfoliação e glaucoma pseudo-esfoliação (p<0,05, para todos). As comparações por pares do grupo síndrome de pseudo-esfoliação e do grupo glaucoma pseudo-esfoliação (p<0,05, para todos). As comparações por pares do grupo síndrome de pseudo-esfoliação e do grupo glaucoma pseudo-esfoliação não revelaram diferenças significativas entre esses dois grupos quanto à espessura corneana central, à profundidade aquosa, à profundidade da câmara anterior e aos valores de espessura da lente (p>0,017, para cada um).
Conclusões: Os casos de glaucoma pseudo-esfoliação e de síndrome de pseudo-esfoliação apresentaram lente mais espessa, menor profundidade aquosa, menor profundidade da câmara anterior e espessura corneana central mais fina do que os indivíduos normais. No entanto, nenhum dos parâmetros do segmento anterior foi diferente entre os indivíduos do grupo síndrome de pseudo-esfoliação e do grupo glaucoma pseudo-esfoliação.
Descritores: Segmento anterior do olho; Córnea; Lentes; Glaucoma; Síndrome de exfoliação
INTRODUCTION
Pseudoexfoliation syndrome (PXS) is a systemic disease that is frequently seen in older people(1). Ocular involvement of the disease is generally bilateral but asymmetric. PXS mainly causes pseudoexfoliation glaucoma (PXG), which is more progressive and serious than primary open-angle glaucoma (POAG)(2-5). In addition to glaucoma, cataract formation, poor pupillary dilatation, blood-aqueous barrier impairment, zonular dialysis, lens subluxation, and both intraoperative and postoperative complications of cataract surgery are frequently associated with PXS(2-6). Although its pathogenesis is not completely clear, the disease is characterized by the accumulation of pseudoexfoliation material (PXM) in many tissues of the body, including the eye(7-9).
New technologies in anterior segment imaging systems provide useful information for the diagnosis of many diseases(10,11). Noncontact biometers use low-coherence reflectometry methods and can be used for some anterior segment measurements, such as central corneal thickness (CCT), aqueous depth (AD), anterior chamber depth (ACD), lens thickness (LT), axial length (AL), K1 and K2 keratometry, and white to white distance (WTW)(12,13).
In this study, we aimed to compare the anterior segment parameters PXS and PXG patients with those in normal subjects by optical biometry.
METHODS
Subjects
Fifty eyes of 50 patients with PXS, 50 eyes of 50 patients with PXG, and 50 eyes of 50 healthy subjects who had undergone ophthalmological examination in a tertiary eye hospital between September 2014 and September 2016 were included in this prospective cross-sectional study. PXG and PXS patients received their diagnosis at the time of the study and had never used any antiglaucoma medications. The control subjects were chosen from patients who had applied for routine ophthalmological examination at the same hospital. The study was approved by the Ethics Committee of Ankara Numune Training Hospital. All procedures performed in studies involving human subjects were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. All participants were Turkish Caucasians.
Examinations
All of the participants had detailed ophthalmologic examinations including best-corrected visual acuities with Snellen charts, biomicroscopic and fundoscopic examinations, and intraocular pressure (IOP) measurements with Goldmann applanation tonometer. The patients with PXS and PXG underwent retinal nerve fiber layer thickness analysis and optic disc topography by Heidelberg Retina Tomography 3 (HRT 3) and visual field analysis by Humphrey automated perimeter.
PXS was defined as the presence of PXM deposits on the edge of the pupil and/or lens capsule during the biomicroscopic examination, an IOP less than 21 mmHg, and no sign of glaucomatous optic nerve damage on fundus and/or visual field examination. PXG was diagnosed if the patient had typical characteristics of PXS with a grade 3 to 4 open angle according to the Shaffer angle grading system as well as glaucomatous optic disc changes (vertical cup to disc [C/D] ratio >0.3, C/D asymmetry >0.2 between the eyes, focal notching, and localized nerve fiber layer defects) with compatible visual field defects and documented IOP ≥22 mmHg. The visual field was considered glaucomatous when one of the following criteria was met: a glaucoma hemifield test outside the normal limits; a band of three or more non-edge points approved with a p<0.05 probability of normality, one of which with p<0.01 and none of which was contiguous with the blind spot; or a pattern standard deviation value with p<0.05. Visual field results had to be reliable based on false positive rates ≤25% and false negative rates and fixation losses ≤33%. We included PXG patients in our study with stage 1 (mean deviation ≤-6 dB) or stage 2 -6 to -12 dB) glaucoma according to the Hodapp-Parrish-Anderson grading scale. Subjects were excluded if they had diabetes mellitus; cataract; uveitis; corneal diseases that may cause irregular astigmatism, such as keratoconus or spherical and cylindrical refractive errors >±1.00 diopter (D), or a history of previous intraocular surgery or trauma; if they were younger than 30 years; or if they were using antiglaucoma drops. Eyes with stage 3, 4, or 5 glaucoma (by the Hodapp-Parrish-Anderson grading scale) were excluded. Control subjects were 30 years old or older, had no evidence of exfoliative material in the intraocular tissues, had an IOP<22 mmHg, had normal-appearing optic nerves, and had no ocular problems other than spherical or cylindrical refractive errors ≤±1.00 D, blepharitis, or allergic conjunctivitis.
AL, CCT, AD, ACD LT, K1, and K2 values and WTW were measured by the same experienced physician (M.K.) with optical biometry (LenStar LS 900, Haag-Streit Diagnostics, Koeniz, Switzerland) at least 2 hours after IOP measurement.
Statistical analysis
Study data were analyzed with the Statistical Package for Social Sciences (SPSS) for Windows, version 22.0 (SPSS, Chicago, IL, USA). Descriptive statistics were presented as means ± standard deviation, frequency distributions, and percentages. The chi-square test was used for analysis of categorical variables. Normal distribution of variables was tested by visual (histogram and probability graphs) and analytical (Kolmogorov-Smirnov/ Shapiro-Wilk Test) methods. Equality of variances was checked by the Levene test. One-way analysis of variance, Welch analysis of variance, and the Kruskal-Wallis test were used to determine whether there were any significant differences among the three groups. A probability level of p<0.05 was considered to indicate statistical significance.
RESULTS
The mean age of the 25 male (50%) and 25 female (50%) patients with PXS was 62.18 ± 6.21 years (range, 35-70 years); the mean age of the 28 male (56%) and 22 female (44%) patients with PXG was 61.80 ± 6.62 years (range, 35-70 years); and the mean age of the 29 male (58%) and 21 female (42%) control subjects was 59.40 ± 6.89 years (range, 47-70 years). There were no statistically significant differences between the groups in mean age or sex ratio (p>0.05). Patient characteristics are shown in table 1.
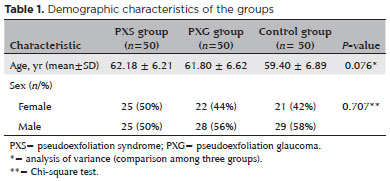
The mean values of CCT, AD, ACT, and LT were 525.94 ± 28.55 µm, 2.65±0.33 mm, 3.18 ± 0.34 mm, and 4.43 ± 0.41 mm in the PXS group; 521.84 ± 39.97 µm, 2.77 ± 0.42 mm, 3.30±0.43 mm, and 4.44±0.42 mm in the PXG group; and 545.18 ± 33.51 µm, 3.07 ± 0.34 mm, 3.60 ± 0.33 mm, and 3.96 ± 0.45 mm in the control group, respectively. Pairwise comparisons of the PXS group and the PXG group found no significant differences between these two groups in CCT, AD, ACD, or LT values (p>0.017). The mean values of AL, K1, and K2 values, and WTW were similar in all groups (p>0.05). Comparison of anterior segment parameters obtained by optical biometry in the study groups is shown in table 2.
DISCUSSION
PXS affects all parts of the eye, especially the anterior segment, by the accumulation of PXM(2-5). It is a risk factor for many clinical entities, such as small pupil, cataract, and zonular laxity, but the most frequent and important clinical condition associated with the syndrome is PXG, which is more aggressive than POAG(2-5). Although the progression from PXS to PXG is not clear, it may be related to other factors besides IOP, such as increased vulnerability of the optic nerve(7). In this study, our aim was to compare the anterior segment parameters of PXS patients, PXG patients, and normal subjects by optical biometry. We especially included patients with PXG in the early stages of glaucoma because of the possible relation between the severity of PXG and the increased presence of PXM(14), which may strongly affect the structure of the anterior segment due to increased zonular laxity. All of the patients with PXG had newly diagnosed glaucoma, and none of them had used any antiglaucoma agents. Our primary aim was to determine differences between patients with PXS and those in the early stages of PXG.
Thin CCT is known to be a risk factor for almost every type of glaucoma(15). Many studies have reported CCT in patients with PXS and PXG(16-19). Doganay et al.(16) investigated anterior segment parameters in patients with PXS and PXG, as in our study, but using a Pentacam-Scheimpflug imaging system that was different from ours. They did not find any significant differences in CCT between patients with PXS or PXG and normal subjects. Ozcura et al.(17) measured CCT values by ultrasonic pachymeter in patients with PXS and PXG and compared them with values in normal subjects. They stated that thin CCT might cause a rapid progression of glaucoma both as an independent risk factor and as a result of artificially lowered IOP readings. In our study, both PXS and PXG patients had significantly thinner CCT values than control subjects, but there was no significant difference between the mean CCT values of PXG and PXS patients. Unlike our study and the studies of Doganay et al.(16) and Ozcura et al.(17), Tomaszewski et al.(18) found significantly thinner CCT values in PXG patients than in PXS patients and control subjects, a finding that suggests CCT is a risk factor for the progression from PXS to PXG. They also found lower values of corneal endothelial cell density in patients with PXG and PXS than in control subjects, indicating corneal involvement of the disease(18,19). Keel et al.(20) investigated diurnal variation of CCT in addition to IOP in PXS patients without glaucoma and stated that a reduction in CCT between 8 am and 5 pm might be partially related to the reduction in IOP during the same period. Gorezis et al.(21) compared mean CCT in patients with PXG with that in patients with POAG and ocular hypertension (OHT). They found significantly thinner CCT values in PXG patients than in those with POAG and OHT. Their result supports the result of Tomaszewski et al.(18) and identifies CCT as a risk factor for glaucoma, especially for PXG.
ACD and AD are the other important anterior segment parameters showing significant differences between the groups in our study. We found significantly shallower ACD and smaller AD values in the PXS and PXG groups, but no significant differences were found between the PXS and PXG groups. Doganay et al.(16) found significantly lower ACD values in PXG patients than in control subjects by using Pentacam-Scheimpflug imaging, but no significant differences were found between PXS patients and control subjects. Atalay et al.(22) investigated the mean lens-iris distance in PXS patients and normal subjects and found lower values in PXS patients. The results of Doganay et al.(16) and Atalay et al.(22), as well as the results of our study, should be related to zonular laxity in pseudoexfoliation. Ritch et al.(23) investigated zonular appearance in PXS patients by ultrasound biomicroscopy and showed that patients with moderate to severe PXS had statistically significantly higher scores than subjects with early PXS or no PXS. Zonular laxity may predispose to phacodonesis and narrow or closed iridocorneal angles in PXG and PXS(24-26). Prostaglandin analogs also cause zonular laxity and alterations in ACD(27). Because of this, we included only new PXG patients who had never used antiglaucoma drops prior to our study.
We also found significant differences in LT between the PXG, PXS, and control groups. Our PXS and PXG patients had thicker lenses than normal subjects, but there was no statistically significant difference between the PXS and PXG groups in LT. Yavas et al.(28) found no statistically significant difference in LT between patients with PXS and normal subjects.
In addition to CCT, pseudoexfoliation may also affect the dimensions of the cornea and AL. Ozcura et al.(17) found no significant differences in K1, K2, mean K readings, and AL between PXS patients, PXG patients, and normal subjects, similar to our results.
The major limitations of the current study are the relatively small number of patients and its cross-sectional nature. The strong points of the study are that all our PXG patients had newly diagnosed glaucoma and none of them had used any antiglaucoma agents that might have affected the results.
In conclusion, pseudoexfoliation, even in PXS patients or those in the early stages of PXG, had some effects on anterior segment parameters, especially CCT, ACD, AD, and LT, and can result in thinner cornea, shallower AD, and thicker lens. Our hypothesis was that some factors might affect anterior chamber parameters in the progression of PXS to PXG, such as the amount of accumulated PXM, which was postulated to affect the anterior chamber structures. The hypothesis predicts that there would be differences between PXS patients and those in the early stages of PXG in anterior chamber parameters. However, because of the cross-sectional nature of this study, a causal relationship between PXM and alterations in anterior chamber parameters cannot be inferred and the progression to glaucoma cannot be evaluated. Further prospective, longitudinal, comprehensive studies are needed to elucidate changes in anterior chamber parameters in patients with PXS and PXG, and to investigate whether these parameters may affect the progression from PXS to PXG.
REFERENCES
1. Holló G. Exfoliation syndrome and systemic cardiovascular diseases. J Glaucoma. 2014;23(1):9-11.
2. Keel S, Malesic L, Chan SP. Diurnal variation in central corneal thickness and intraocular pressure in eyes with pseudoexfoliation syndrome without glaucoma. Indian J Ophthalmol. 2014;62(11): 1072-6.
3. Rasmussen CA, Kaufman PL. The trabecular meshwork in normal eyes and in exfoliation glaucoma. J Glaucoma. 2014;23(8):15-9.
4. Tarkkanen AH, Kivelä TT. Mortality in primary open-angle glaucoma and exfoliative glaucoma. Eur J Ophthalmol. 2014;24(5):718-21.
5. Musch DC, Shimizu T, Niziol LM, Gillespie BW, Cashwell LF, Lichter PR. Clinical characteristics of newly diagnosed primary, pigmentary and pseudoexfoliative open-angle glaucoma in the Collaborative Initial Glaucoma Treatment Study. Br J Ophthalmol. 2012;96(9):1180-4.
6. Sangal N, Chen TC. Cataract surgery in pseudoexfoliation syndrome. Semin Ophthalmol. 2014;29(5-6):403-8.
7. Anastasopoulos E, Founti P, Topouzis F. Update on pseudoexfoliation syndrome pathogenesis and associations with intraocular pressure, glaucoma and systemic diseases. Curr Opin Ophthalmol. 2015;26(2):82-9.
8. Dewundara S, Pasquale LR. Exfoliation syndrome: a disease with an environmental component. Curr Opin Ophthalmol. 2015;26(2): 78-81.
9. Zenkel M, Schlötzer-Schrehardt U. The composition of exfoliation material and the cells involved in its production. J Glaucoma. 2014; 23(8):12-4.
10. Shankar H, Taranath D, Santhirathelagan CT, Pesudovs K. Anterior segment biometry with the Pentacam: comprehensive assessment of repeatability of automated measurements. J Cataract Refract Surg. 2008;34(1):103-13.
11. Galletti JD, Ruiseñor Vázquez PR, Minguez N, Delrivo M, Bonthoux FF, Pförtner T, et al. Corneal asymmetry analysis by pentacam scheimpflug tomography for keratoconus diagnosis. J Refract Surg. 2015;31(2):116-23.
12. Rohrer K, Frueh BE, Walti R, Clemetson IA, Tappeiner C, Goldblum D. Comparison and evaluation of ocular biometry using a new noncontact optical low-coherence reflectometer. Ophthalmology. 2009;116(11):2087-92.
13. Rabsilber TM, Jepsen C, Auffarth GU, Holzer MP. Intraocular lens power calculation: clinical comparison of 2 optical biometry devices. J Cataract Refract Surg. 2010;36(2):230-4.
14. Gottanka J, Flügel-Koch C, Martus P, Johnson DH, Lütjen-Drecoll E. Correlation of pseudoexfoliative material and optic nerve damage in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 1997; 38(12):2435-46.
15. Wang SY, Melles R, Lin SC. The impact of central corneal thickness on the risk for glaucoma in a large multiethnic population. J Glaucoma. 2014;23(9):606-12.
16. Doganay S, Tasar A, Cankaya C, Firat PG, Yologlu S. Evaluation of Pentacam-Scheimpflug imaging of anterior segment parameters in patients with pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Clin Exp Optom. 2012;95(2):218-22.
17. Ozcura F, Aydin S, Dayanir V. Central corneal thickness and corneal curvature in pseudoexfoliation syndrome with and without glaucoma. J Glaucoma. 2011;20(7):410-3.
18. Tomaszewski BT, Zalewska R, Mariak Z. Evaluation of the endothelial cell density and the central corneal thickness in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Ophthalmol. 2014; 2014:123683. doi: 10.1155/2014/123683.
19. Yazgan S, Celik U, Alagöz N, Taş M. Corneal biomechanical comparison of pseudoexfoliation syndrome, pseudoexfoliative glaucoma and healthy subjects. Curr Eye Res. 2015;40(5):470-5.
20. Keel S, Malesic L, Chan SP. Diurnal variation in central corneal thickness and intraocular pressure in eyes with pseudoexfoliation syndrome without glaucoma. Indian J Ophthalmol. 2014;62(11): 1072-6.
21. Gorezis S, Christos G, Stefaniotou M, Moustaklis K, Skyrlas A, Kitsos G. Comparative results of central corneal thickness measurements in primary open-angle glaucoma, pseudoexfoliation glaucoma, and ocular hypertension. Ophthalmic Surg Lasers Imaging. 2008;39(1):17-21.
22. Atalay E, Tamçelik N, Bilgec MD. Quadrantwise Comparison of lens-iris distance in patients with pseudoexfoliation syndrome and age-matched controls. Cornea. 2016;25(1):95-100.
23. Ritch R, Vessani RM, Tran HV, Ishikawa H, Tello C, Liebmann JM. Ultrasound biomicroscopic assessment of zonular appearance in exfoliation syndrome. Acta Ophthalmol Scand. 2007;85(5):495-9.
24. Crichton AC, Oryschak AF, McWhae JA, Kirker AW, Chacon-Andrade H. Postmortem microscopic evaluation and clinical correlation of a pseudophakic eye with pseudoexfoliation and loss of zonular support. J Cataract Refract Surg. 2007;33(1):162-5.
25. Desai MA, Moon CS, Bretana ME, Ehlies F, Winnick M, Lee RK. Pupillary block glaucoma secondary to phacodonesis in pseudoexfoliation. Ophthalmic Surg Lasers Imaging 2010;9:1-7. doi: 10.3928/15428877-20100215-80.
26. Bosnar D, Kuzmanović Elabjer B, Bušić M, Bjeloš Rončević M, Miletić D, Barać J. Optical low-coherence reflectometry enables preoperative detection of zonular weakness in pseudoexfoliation syndrome. Graefes Arch Clin Exp Ophthalmol. 2010;250(1):87-93.
27. Cankaya AB, Teberik P, Acaroglu G. Alterations in anterior chamber depth in primary open-angle glaucoma patients during latanoprost therapy. Acta Ophthalmol. 2011;89(3):274-7.
28. Yavas GF, Oztürk F, Küsbeci T, Inan UU, Kaplan U, Ermiş SS. Evaluation of the change in accommodation amplitude in subjects with pseudoexfoliation. Eye (Lond). 2009;23(4):822-6.
Submitted for publication:
April 10, 2017.
Accepted for publication:
September 10, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.
Approved by the following research ethics committee: Institutional Review Board of the Ankara Numune Research and Education Hospital (#E-14-294).