INTRODUCTION
Descemet stripping automated endothelial keratoplasty (DSAEK) is the standard surgical treatment for endothelial failure. Its efficacy is indicated by a spectacle-corrected acuity of 20/40 or better in most patients(1,2). The microkeratome coupled to the artificial chamber can produce an endothelial donor lamella (EDL); however, the cutting irregularities and the unpredictability of the depth of the cut are major impediments to the visual outcome of lamellar transplantation(3,4). New models of femtosecond lasers (FS) produce a better corneal lamella quality than the microkeratome(5-9). Lasers with a high frequency and low energy tend to make a corneal cut smoother, more homogeneous, accurate, and reproducible, as smaller intrastromal bubbles are produced, creating smaller cavities in the cornea(5-9). Cell necrosis and inflammation in the cornea increase as the energy used by FS increases(5-9). There is a direct relationship between the frequency and amount of energy required to form a shooting corneal lamella. With a lower frequency, more energy is required to manufacture the lamella. In addition, with more energy, the size of the intrastromal bubbles and cavities generated by each shot increases, which decreases the laser's precision(5-9). Theoretically, a better visual outcome can be achieved with a thinner and more regular corneal donor lamella produced by a low-energy and high-frequency FS. The objective of this report is to describe, for the first time in Brazil, the creation of an EDL with a low energy and high frequency FS and its subsequent use in vivo.
CASE REPORT
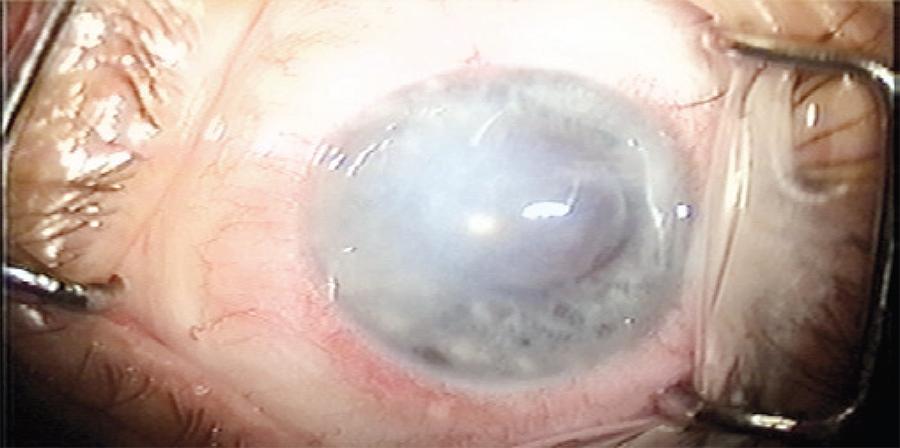
A 59-year-old female presented with an endothelial failure in the left eye (LE) after phacoemulsification and intraocular lens implantation performed 9 years previously. Figure 1 presents a preoperative photograph of her LE. Her best-corrected visual acuity (BCVA, with glasses) was a finger count at 1 m. The central corneal thickness (CCT) was 730 µm, as measured by ultrasonic pachymetry (P55, Paradigm®, USA). Following the preoperative examinations, she was subjected to DSAEK.
An EDL was prepared with a low energy and high frequency FS (LDV®, Ziemer® Ophthalmic System AG, Port, Switzerland). This laser operates with a pulse frequency in the megahertz range, with highaperture optics, with a per pulse time exposure of 250 fs, and a spot diameter of 2 µm. The shots were overlapped to avoid leaving untreated areas. The energy per pulse was 30 nJ(5).
For proper laser application, the donor cornea was first attached to the Ziemer® anterior chamber, the epithelium was removed, and CCT was measured by ultrasonic pachymetry. The donor cornea was then flattened using the head of the laser. The preoperative donor endothelium cell count was 2835 cells/mm2, and the intraoperative CCT was 590 µm. The anterior donor lamella was planned to have a 10-mm diameter and a 500-µm thickness, theoretically leaving a 90-µm EDL. After laser treatment, the cornea was manually trephined from the endothelial side using an 8-mm trephine blade (Katena®Products, Denville, NJ, USA). Next, the posterior and anterior lamellae were separated by holding the edge of the posterior lamella with forceps and gently sliding off the anterior lamella using the tip of a Merocel® sponge (Figure 2).
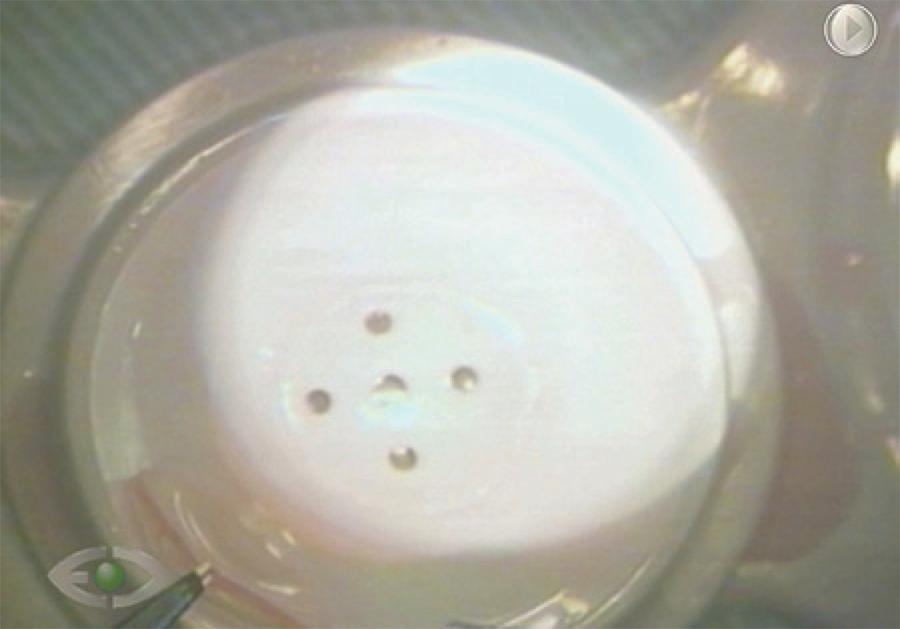
Figure 2 Deep anterior donor cornea lamellae (thicker) being removed. Endothelial donor lamellae (thinner), from same cornea, remains on the bottom with the endothelium side up after cutting with an LDV® femtosecond laser
The surgeon (WN) performed a standard DSAEK procedure in the receptor eye after peribulbar anesthesia, as described previously(2). In brief, the anterior chamber was filled with air through the paracentesis and a reverse Sinskey hook was used to remove an approximately 8-mm diameter section of the central endothelium and Descemet membrane, previously marked on the host epithelium, through the main 2.4-mm clear corneal incision. Afterwards, the main incision was enlarged with a 5.2-mm surgical blade to insert the endothelial lamella with a Busin® glide (Moria®, France). The anterior chamber was maintained with air throughout the procedure. The EDL was positioned over the receptor corneal epithelium using a cannula with smooth movements. The main incision was sutured with a single 10-0 mononylon stitch. The patient remained in the supine position for 2 h to promote proper EDL adhesion when the air was partially removed and the anterior chamber was filled with balanced saline solution.
After surgery, topical moxifloxacin hydrochloride 0.5% (Vigamox®, Alcon®, Brazil) was prescribed four times per day for 1 week and prednisolone acetate 1% (Predfort®, Allergan®, Brazil) every 3 h for 1 week, then six times per day for 1 week, which was decreased thereafter by one application per day each week.
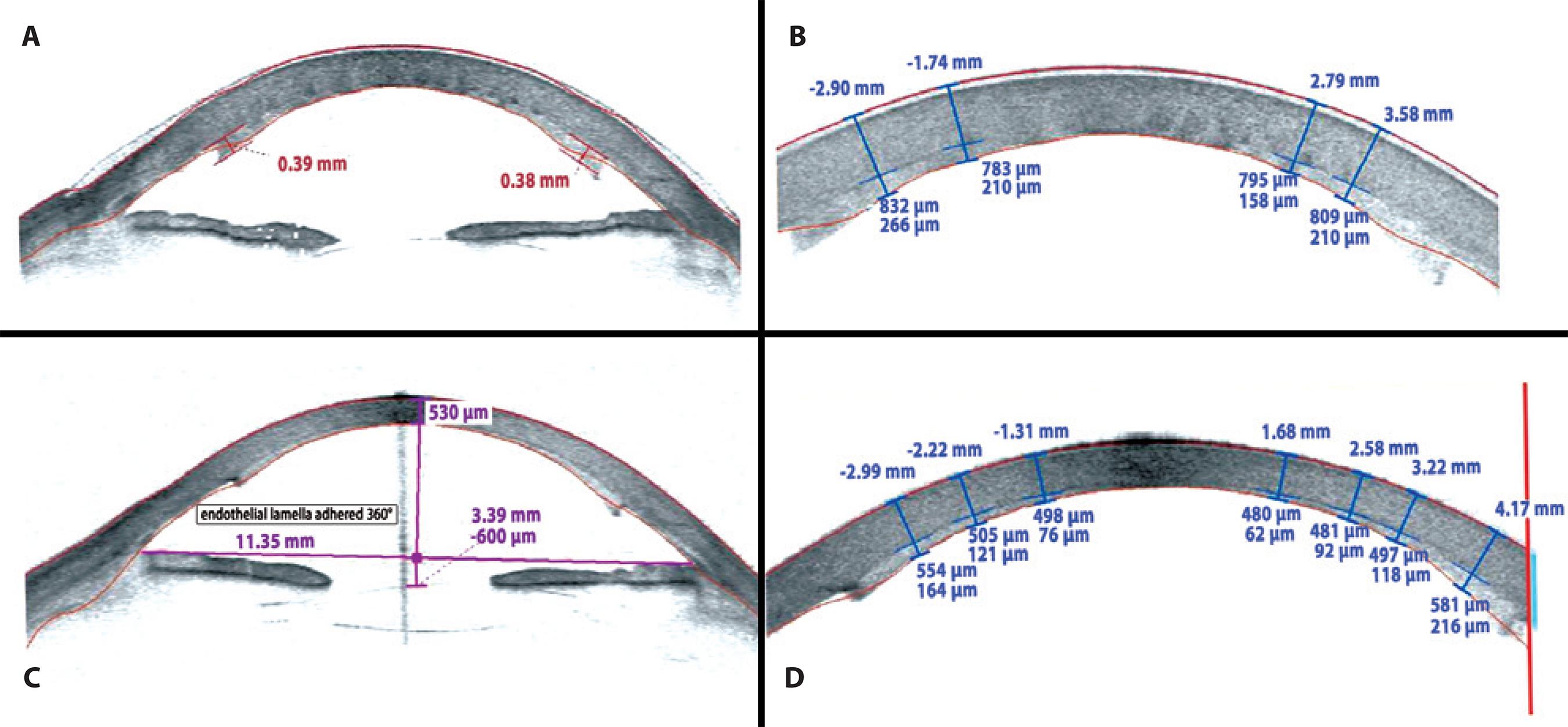
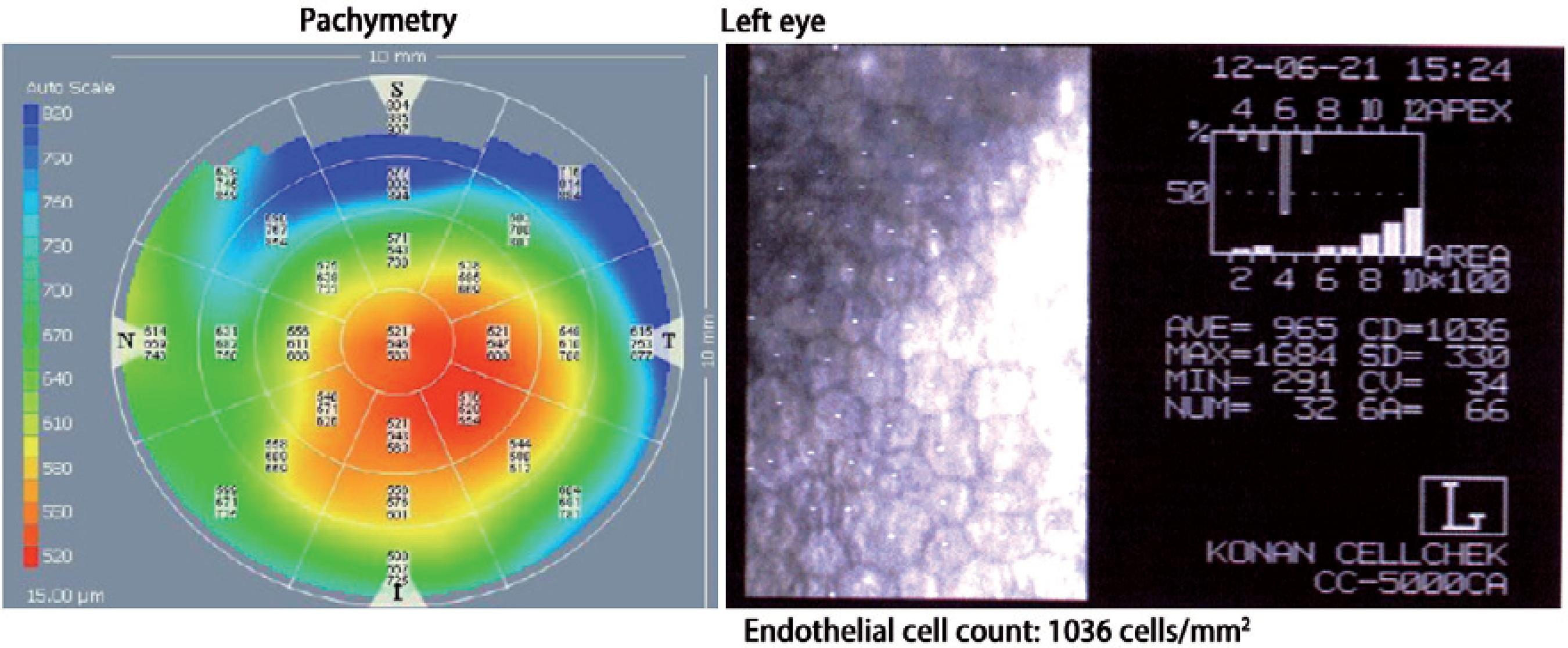
On the first postoperative day, the patient had an uncorrected visual acuity (UCVA) of 20/400 in LE. After 1 month, her BCVA was 20/150 (+1.50 -2.50 × 15°). In the third postoperative month, she had an UCVA of 20/150 and her BCVA improved to 20/30 with a refraction of +075 -2.75 × 120°. The central and paracentral endothelial lamella thickness was measured with the Visante®OCT (Zeiss®, Germany) at 158 µm in the first postoperative month and 62 µm after the third postoperative month. Figure 3 A and 3 B show the Visante® OCT after 1 month whereas figure 3 C and 3 D show the same after 3 months. Figure 4 presents the global pachymetric map and endothelial cell count in the third postoperative month. Global pachymetric measurements with the Visante® OCT were within the normal range, with 521 µm for CCT (Figure 4). The endothelial cell count in the third postoperative month was 1036 cells/mm2 (Figure 4).
DISCUSSION
Bullous keratopathy remains one of the leading causes worldwide for corneal transplantation(10). Many surgeons consider DSAEK as the treatment of choice for this condition(1,2). According to the potential vision of the eye, a thinner EDL will result in a better final visual acuity. The endothelial cell count declined significantly, but could maintain a viable tissue. The EDL was set to 90 µm without epithelium, and the effective EDL was 62 µm. The desired thinner thickness was calculated on the basis of the full thickness of the central cornea after removing it from the Optisol®and placing it in the artificial anterior chamber, which certainly had edema. This shows a certain predictability for this technique using this laser. The primary reasons for this small difference may be the influence of pressure within the artificial anterior chamber at the time of preoperative measurements, pachymetric measure after flattening the cornea with the laser to construct the EDL, and the preoperative corneal edema and laser safety margin. These factors should be considered by the surgeon and examined in future studies. In another in vitro study(9) that used the same FS used in the present study, 5 corneas were cut to produce EDLs with a 70-μm thickness and the mean central posterior stromal thickness of the cut corneas was 60.6 μm (range, 43-72 μm). FS can help spread DSAEK when creating an automated ultrafine EDL and facilitate DSAEK, especially if eye banks can send a pre-cut EDL. The disadvantage is the high cost of FS.
Here, we report the production of an ultrathin EDL using a low energy and high frequency FS. We also report its use in vitro and the outcomes observed 3 months after surgery in terms of the evolution of EDL, the total corneal thickness (including the global pachymetric mapping), the visual acuity, and the endothelial cell count.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

