

Leonardo Leivas1; Fernando Procianoy1,2; Rafaela C. Almeida1; Marina M. Pakter4; Marcelo V. Fabris2; Rodrigo L. Lindenmayer2; Fábio Lavinsky3; Daniel Lavinsky1,2; Helena M. Pakter1,2
DOI: 10.5935/0004-2749.2025-0181
ABSTRACT
PURPOSE: To assess the one-year impact of prostaglandin analog (travoprost) on periorbital appearance and lower eyelid horizontal tension, compared with the contralateral eye treated with Pascal selective laser trabeculoplasty.
METHODS: This nested case-control study was derived from a non-inferiority randomized clinical trial comparing Pascal selective laser trabeculoplasty efficacy with travoprost in fellow eyes over 12 months. One eye received daily travoprost, while the contralateral eye underwent Pascal selective laser trabeculoplasty. Lower eyelid horizontal tension was measured using a validated digital imaging method, and prostaglandin-associated periorbitopathy signs were graded by masked observers. Statistical analyses included generalized estimating equations and the Mann–Whitney U test for nonparametric data, with p<0.05 considered significant.
RESULTS: Ten patients met the inclusion criteria for this subanalysis. Travoprost-treated eyes had significantly lower mean lower eyelid distension compared with Pascal selective laser trabeculoplasty-treated eyes (4.32 mm vs. 5.02 mm; mean difference: 0.70 mm; p<0.001). Prostaglandin-associated periorbitopathy scores were significantly higher in the travoprost group, reflecting more pronounced changes in ptosis, periorbital hyperpigmentation, and eyelid retraction.
CONCLUSIONS: Chronic use of prostaglandin analogs is associated with notable periorbital changes and increased lower eyelid tension, potentially affecting aesthetics and ocular function. Pascal selective laser trabeculoplasty may offer a safer profile for preserving periorbital anatomy while maintaining effective intraocular pressure control. Laser trabeculoplasty should be considered as an initial treatment when appropriate to minimize cosmetic and functional changes from chronic topical therapy.
Keywords: Prostaglandin analogs; Selective laser trabeculoplasty; Periorbitopathy; Eyelid tension; Glaucoma treatment
INTRODUCTION
Glaucoma comprises a group of diseases causing retinal ganglion cell loss, nerve fiber layer thinning, optic nerve excavation, and progressive visual field loss(1). It is the leading cause of irreversible blindness worldwide, affecting millions of people. Global prevalence is projected to rise from 60 million in 2010 to 111.5 million by 2040 due to aging populations(2,3). In Brazil, prevalence among adults over 40 years ranges from 2% to 3%, increasing with age(4).
Elevated intraocular pressure (IOP) is the main modifiable risk factor for glaucoma development and progression. Reducing baseline IOP by 30%–50% is associated with slower disease progression(5). Prostaglandin F2α analogs (PGAs) are first-line pharmacologic treatments due to potent IOP-lowering effects and once-daily dosing. They enhance uveoscleral outflow, reducing IOP by approximately 25%–35% from baseline(6).
Selective laser trabeculoplasty (SLT) offers additional benefits, including independence from patient adherence, minimal invasiveness, and low complication rates(7–9). Recent trials, such as SLT/Med and LiGHT, show SLT can achieve 20%–35% IOP reduction comparable with prostaglandins, with lasting effects and fewer side effects; 74% of patients remained medication-free for three years in the LiGHT trial(10).
Our group recently compared Pascal SLT (PSLT), a computer-guided variant of SLT, with travoprost for ocular hypertension (OH) and primary open-angle glaucoma (POAG). Both treatments demonstrated similar IOP reductions over 1 yr, supporting PSLT as a viable alternative (ahead of publication)(11).
Despite their effectiveness, chronic PGA use is associated with prostaglandin-associated periorbitopathy (PAP), including hyperpigmentation, eyelash changes, deepening of the upper eyelid sulcus, ptosis, horizontal tightening, palpebral fissure narrowing, and lower eyelid stiffness, potentially affecting aesthetics and ocular function(12–14).
This study aimed to evaluate the impact of 1-yr travoprost use on eyelid laxity and periorbital aesthetics and function, using a within-subject comparison with PSLT-treated eyes.
METHODS
This nested case-control study was derived from a non-inferiority randomized clinical trial assessing PSLT efficacy (ahead of publication). The study was approved by HCPA (CAAE: 44489320.7.0000.5327) and registered at ClinicalTrials.gov (NCT 05241938). The study adheres to STROBE guidelines(15).
Ten patients met the inclusion criteria. One eye received daily travoprost, and the contralateral eye underwent PSLT. After 1 yr, lower eyelid tension was measured via a validated digital imaging method, and PAP signs were graded by masked observers.
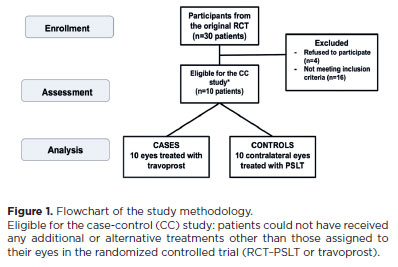
Eligible patients were adults with OH or mild POAG, enrolled from September 2021 to August 2023. Following a one-month washout, eyes were randomized to PSLT or travoprost for 12 months. Exclusion criteria included additional ocular treatments, early discontinuation of travoprost, need for treatment adjustments, or prior eyelid anomalies unrelated to PAP or strabismus (Figure 1). Informed consent was obtained from all participants. Research complied with the Declaration of Helsinki, CNS Resolution 466/2012, and the LGPD.
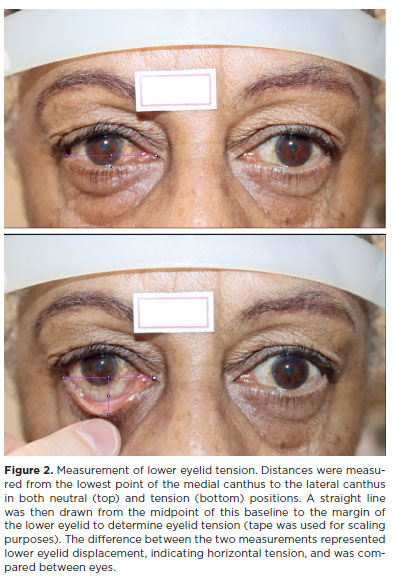
Lower eyelid tension was assessed using a validated digital technique(16). Standardized photographs were taken with patients in primary gaze. Vertical traction was applied to the lower eyelids, and measurements were made using ImageJ 1.33μ software. Horizontal eyelid displacement represented eyelid tension (Figure 2).
PAP signs were independently graded by two examiners for hyperpigmentation, eyelash changes, eyelid alterations (ptosis or retraction), and conjunctival/scleral changes. Scores ranged from 0 (no PAP) to 4 (all signs present).
Data were managed in REDCap and analyzed using SPSS 11.0 (IBM Corp., Armonk, New York). Categorical variables are presented as frequencies and percentages with 95% confidence intervals. Comparisons used generalized estimating equations for repeated measures and Mann–Whitney U tests for nonparametric data. Statistical significance was set at p<0.05.
RESULTS
Ten patients met the inclusion criteria for this case-control study. The mean age of participants was 60.2 ± 9 yr, with the majority being women (80%) and 40% identifying as White. Prior to the washout period, patients were using one or two hypotensive eye drops. Most were on beta-blockers (nine patients), while two were using prostaglandin analogs (Table 1).
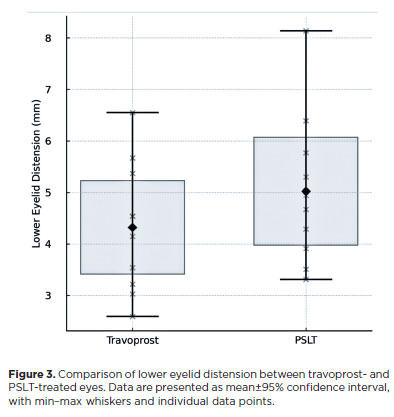
Patients treated with travoprost exhibited a mean reduction of 0.70 mm in the amplitude of lower eyelid distension compared with eyes treated with PSLT. The average eyelid tension in travoprost-treated eyes was 4.32 mm, whereas laser-treated eyes showed an average tension of 5.02 mm. Statistical analysis revealed a significant difference between the two groups (p<0.001; Figure 3).
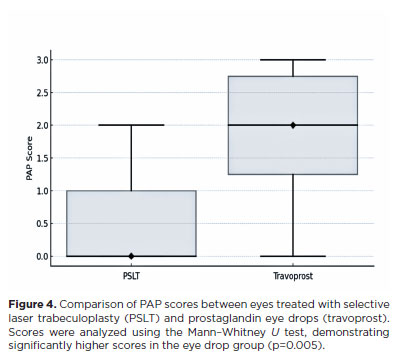
As expected, higher PAP scores were more frequently observed in travoprost-treated eyes than in laser-treated eyes. Score 0, corresponding to no PAP changes, was observed in only one eye in the travoprost group. Statistical analysis indicated that scores in eyes treated with eye drops were significantly higher than in eyes treated with PSLT (p=0.005, nonparametric Mann–Whitney U test, Figure 4). In two patients with prior bilateral PGA use, no signs of PAP were observed in the laser-treated eye after one year, while the travoprost-treated eye exhibited persistent periorbital changes. Travoprost-treated eyes could be identified in 70% of cases.
DISCUSSION
Since the early 2000s, the clinical spectrum of PAP has become increasingly well-defined. Initially described as a deepening of the upper eyelid sulcus, the syndrome is now recognized to include ptosis, dermatochalasis, orbital fat atrophy, hyperpigmentation, and eyelid tightness(17). This historical perspective underscores the importance of monitoring the cosmetic and functional consequences of chronic PGA use.
Our study demonstrates that after just 1 yr of prostaglandin eye drop use, signs of PAP were readily noticeable, along with increased lower eyelid tension, compared with the contralateral eye treated with PSLT. This study contributes to the literature by objectively quantifying eyelid tension, an underexplored functional parameter in PAP assessment.
Increased lower eyelid tension may lead to ocular and aesthetic complications. Eyelid retraction can expose more of the sclera, altering appearance and potentially causing ocular surface exposure and dry eye symptoms due to increased tear evaporation. Impaired lacrimal drainage may result in epiphora. Aesthetic changes may create a fatigued or aged appearance, negatively affecting self-esteem. Furthermore, horizontal eyelid tightening can cause malposition and, in severe cases, affect intraocular pressure measurement(18).
Previous studies have highlighted the aesthetic and functional concerns associated with prolonged PGA use(12,19). SLT, by contrast, is a safe and effective alternative for glaucoma management. Research shows that SLT not only lowers IOP effectively but also reduces the risk of adverse side effects compared with pharmacological therapy. SLT has also proven cost-effective, including within the Brazilian Unified Health System (SUS)(20,21), optimizing resources and improving outcomes. Consequently, many physicians and guidelines now consider SLT a viable first-line option for newly diagnosed open-angle glaucoma. It is important to note that PSLT should not be regarded as inherently protective; rather, it avoids prostaglandin exposure, which drives periorbital changes. Therefore, the observed differences are best understood as consequences of prostaglandin-related adverse effects rather than benefits of PSLT.
This study has several limitations. The small sample size, although sufficient for primary treatment comparison, prevented stratified analyses by age, sex, or prior prostaglandin exposure and may limit generalizability. Two participants had prior PGA use, potentially influencing baseline periorbital features. Nevertheless, the absence of PAP signs in the laser-treated eyes of these patients after 12 months supports the reversible nature of PGA-induced changes, consistent with reports of PAP regression following discontinuation. Complete masking was also impossible; although observers grading PAP were blinded, some clinical signs may have been recognized, introducing subtle bias. Additionally, despite using a validated method, eyelid tension assessment can be examiner-dependent and influenced by age, skin elasticity, sun exposure, and ethnicity, potentially affecting measurement consistency.
Understanding prostaglandin-associated side effects is essential in glaucoma management. Physicians should discuss risks and benefits with patients and consider SLT as a safer alternative. Regular monitoring of intraocular pressure and periorbital features is necessary to optimize ocular health and treatment outcomes. While controlling glaucoma progression is paramount, maintaining periorbital appearance and function can enhance self-esteem, comfort, and treatment adherence.
In conclusion, this study shows that within one year, chronic prostaglandin analog use can induce measurable functional and aesthetic changes consistent with PAP. Despite limitations in sample size and methodology, these findings provide new insights into the broader consequences of PGA therapy and highlight the importance of individualized glaucoma management that considers both visual prognosis and quality of life.
ACKNOWLEDGMENTS
This study was supported by the Hospital de Clínicas de Porto Alegre, RS, Brazil. The authors sincerely acknowledge the employees and staff members of HCPA, as well as the residents and fellows of the Hospital de Clínicas de Porto Alegre, for their valuable assistance during data collection. We also thank the patients who consented to the use of their clinical images for this study.
AUTHORS’ CONTRIBUTIONS
Significant Contribution to Conception and Design: Helena M Pakter, Fernando Procianoy. Data Acquisition: Leonardo Leivas, Rafaela C. Almeida, Marina M. Pakter, Marcelo V. Fabris, Helena M. Pakter. Data Analysis and Interpretation: Leonardo Leivas, Fernando Procianoy, Fábio Lavinsky, Helena M. Pakter. Manuscript Drafting: Leonardo Leivas, Helena M. Pakter. Significant Intellectual Content Revision of the Manuscript: Fernando Procianoy, Rodrigo L. Lindenmayer, Fábio Lavinsky, Daniel Lavinsky, Helena M. Pakter. Final Approval of the Submitted Manuscript: Leonardo Leivas, Fernando Procianoy, Rafaela C. Almeida, Marina M. Pakter, Marcelo V. Fabris, Rodrigo L. Lindenmayer, Fábio Lavinsky, Daniel Lavinsky, Helena M. Pakter. Statistical Analysis: Helena M. Pakter. Obtaining Funding: Helena M. Pakter. Supervision of Administrative, Technical, or Material Support: Helena M. Pakter. Research Group Leadership: Helena M. Pakter.
REFERENCES
1. Munemasa Y, Kitaoka Y. Molecular mechanisms of retinal ganglion cell degeneration in glaucoma and future prospects for cell body and axonal protection. Front Cell Neurosci. 2013;6:60.
2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-7. Comment in: Br J Ophthalmol. 2006;90(3):253-4.
3. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90. Comment in: Ophthalmology. 2015;122(7):e40-1.
4. Sakata K, Sakata LM, Sakata VM, Santini C, Hopker LM, Bernardes R, et al. Prevalence of glaucoma in a South Brazilian population: Projeto Glaucoma. Invest Ophthalmol Vis Sci. 2007;48(11):4974-9.
5. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183-93. Comment in: Lancet. 2018;391(10122):739-40.
6. Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019;210(4):180-7.
7. Zaharia AC, Dumitrescu OM, Radu M, Rogoz RE. Adherence to therapy in glaucoma treatment—a review. J Pers Med. 2022;12(4):514.
8. Sarenac T, Bečić Turkanović A, Ferme P, Gračner T. A review of selective laser trabeculoplasty: “The hype is real”. J Clin Med. 2022;11(13):3879.
9. Zgryźniak A, Przeździecka-Dołyk J, Szaliński M, Turno-Kręcicka A. Selective laser trabeculoplasty in the treatment of ocular hypertension and open-angle glaucoma: clinical review. J Clin Med. 2021;10(15):3307.
10. Wright DM, Konstantakopoulou E, Montesano G, Nathwani N, Garg A, Garway-Heath D, Crabb DP, Gazzard G; Laser in Glaucoma and Ocular Hypertension Trial (LiGHT) Study Group; et al. Visual field outcomes from the multicenter, randomized controlled Laser in Glaucoma and Ocular Hypertension Trial (LiGHT). Ophthalmology. 2020;127(10):1313-21.
11. Almeida R, Lindenmeyer RL, Lavinsky F, Lavinsky D, Picetti E, Silva MO da, et al. Pascal selective laser trabeculoplasty (PSLT) in the treatment of ocular hypertension and open-angle glaucoma compared to travoprost eye drops: a non-inferiority clinical trial. Manuscript submitted for publication. Forthcoming 2025.
12. Procianoy F, Lang PL, Bocaccio JL. Lower eyelid horizontal tightening in prostaglandin associated periorbitopathy. Ophthalmic Plast Reconstr Surg. 2021;37(3 Suppl):S76-S79.
13. Custer PL, Kent TL. Observations on prostaglandin orbitopathy. Ophthalmic Plast Reconstr Surg. 2016 Mar-Apr;32(2):102-5.
14. Kucukevcilioglu M, Bayer A, Uysal Y, Altinsoy HI. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42(2):126-31.
15. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-9.
16. Stuchi DP, Rossato J, Bocaccio FJL, Procianoy F. Intra- and interobserver reliability of a modified distraction test based on digital images to assess lower eyelid horizontal tension. Arq Bras Oftalmol. 2020;83(2):116-20.
17. Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81(8):574-7.
18. Osaki TH, Osaki MH, Ohkawara LE, Osaki T, Gameiro GR, Melo LAS Jr. Possible influence of upper blepharoplasty on intraocular pressure. Ophthalmic Plast Reconstr Surg. 2020;36(4):346-8.
19. Li W, Chen X, Chen S, Lv Z, Tang J, Li N. Changes in prostaglandin-associated periorbital syndrome: a self-controlled and prospective study. Cutan Ocul Toxicol. 2025;44(1):35-42.
20. Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, Ambler G, Bunce C, Wormald R, Nathwani N, Barton K, Rubin G, Buszewicz M; LiGHT Trial Study Group. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-16. Comment in: Lancet. 2019;393(10180):1479-80.
21. Barbosa LEO, Barboza WL, Guedes RAP, Chaoubah A, Hatanaka M. Cost-effectiveness of selective laser trabeculoplasty as a replacement for hypotensive eye drops in the Brazilian public health system. Clinics (Sao Paulo). 2025;80:100650.
Submitted for publication:
July 3, 2025.
Accepted for publication:
October 28, 2025.
Approved by the following research ethics committee: Hospital de Clínicas de Porto Alegre – HCPA (CAAE: 44489320.7.0000.5327).
Data Availability Statement: The datasets produced and/or analyzed in this study can be provided to referees upon request.
Edited by
Editor-in-Chief: Newton Kara-Júnior
Associate Editor: Jayter de Paula
Disclosure of Potential Conflicts of Interest: The authors declare no potential conflicts of interest.