

Antônio Lucas Oliveira Correia1,2; Breno Santos Holanda1; Vital Paulino Costa2; José Paulo Cabral de Vasconcelos2
DOI: 10.5935/0004-2749.2025-0140
ABSTRACT
PURPOSE: Glaucoma is a chronic and progressive disease that requires long-term treatment and continuous monitoring. The Kahook Dual Blade, a device used to perform goniotomy in adults, is designed to improve intraocular pressure control in patients with glaucoma. This study aimed to evaluate the long-term efficacy and safety of kahook dual blade goniotomy in glaucoma patients undergoing cataract surgery over a 36-month follow-up.
METHODS: This was a retrospective case series including 56 eyes from 56 patients with mild-to-moderate primary open-angle glaucoma who underwent phacoemulsification combined with kahook dual blade goniotomy. Mean intraocular pressure values, number of preoperative and postoperative hypotensive eye drops, procedure survival, and complications were evaluated over 36 months. Surgical success was defined as either a reduction in intraocular pressure of ≥20% with intraocular pressure between 6 and 18 mmHg without additional medication or a reduction of ≥1 eye drop with intraocular pressure between 6 and
18 mmHg.
RESULTS: The mean preoperative intraocular pressure decreased from 15.96 ± 2,83) mmHg to 13.14 ± 2,11) mmHg at 36 months, representing a 14.9% reduction (p<0.001). The mean number of eye drops decreased from 1.91 ± 0,75) to 1.34 ± 0,92), a 29.8% reduction (p<0.001). The overall success rate was 69.6% at 36 months.
CONCLUSION: Kahook dual blade goniotomy combined with cataract surgery significantly reduced intraocular pressure and the number of hypotensive eye drops required in patients with mild-to-moderate primary open-angle glaucoma, with a favorable success rate maintained at 36 months.
Keywords: Glaucoma, open-angle/surgery; Gonioscopy/methods; Intraocular pressure/physiology; Lens implantation, intraocular; Phacoemulsification/methods; Trabeculectomy/instrumentation; Treatment outcome
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide, with its prevalence continuing to rise in recent years(1). It is estimated that approximately 112 million people will be affected by the disease by 2040(2). Lowering intraocular pressure (IOP) remains the only proven strategy to slow disease progression(3-5). In recent years, considerable efforts have been made to develop less invasive surgical techniques and devices that can reduce IOP and control glaucoma while minimizing surgical complications and allowing faster postoperative recovery.
Several of these procedures target the trabecular outflow pathway, which has traditionally been used for goniotomy in patients with congenital glaucoma, demonstrating favorable outcomes in IOP control(6). However, the efficacy of goniotomy in adults has been limited, with failures often attributed to the development of goniosynechiae(7,8). Consequently, newer techniques have been introduced to lower IOP with improved efficacy and safety profiles.
The Kahook Dual Blade (KDB; New World Medical, Rancho Cucamonga, California) is a dual-blade surgical knife specifically designed to perform ab interno goniotomy with minimal structural damage(9). The procedure is most often performed in conjunction with cataract surgery. Previous studies have reported IOP reductions ranging from 11% to 36% and a decrease of 11% to 92% in the number of hypotensive eye drops after 12 months of follow-up(10-12).
However, evidence on the long-term outcomes of this procedure remains limited, with most studies restricted to follow-up periods of 12 to 18 months(11-15). The present study therefore aims to evaluate the long-term efficacy and safety of KDB goniotomy combined with cataract surgery over a 36-month follow-up.
METHODS
This was a retrospective case series of patients who underwent phacoemulsification combined with KDB goniotomy at the General Hospital of Fortaleza, Ceará, Brazil, between January 2018 and December 2020. The study protocol was approved by the Ethics Committee of the General Hospital of Fortaleza in January 2023 (CAAE: 64265522.1.0000.5040) and conducted in accordance with the tenets of the Declaration of Helsinki.
Inclusion and exclusion criteria
Eligible patients were older than 18 yr and had a diagnosis of mild-to-moderate primary open-angle glaucoma, defined according to the Hodapp-Parrish-Anderson classification(16). Patients with a mean deviation (MD) better than −6 dB were classified as having mild glaucoma, and those with MD between −6 and −12 dB were classified as having moderate glaucoma. Additional requirements included gonioscopy showing an open angle without signs of prior angle closure and evidence of typical glaucomatous optic nerve damage.
Only patients who underwent phacoemulsification with KDB goniotomy and had a minimum follow-up of 36 months were included. Exclusion criteria were use of systemic medications affecting IOP, intraoperative complications during phacoemulsification, or history of previous ocular surgeries. For patients who underwent bilateral surgery, only the first operated eye was included in the analysis.
Surgical technique
All patients underwent standard cataract surgery with phacoemulsification through a 2.75-mm clear corneal incision, followed by in-the-bag intraocular lens (IOL) implantation under local anesthesia. After IOL placement, 1% acetylcholine was instilled to induce miosis and enhance visualization of angle structures, and the anterior chamber was filled with viscoelastic.
The surgeon was positioned temporally, with the patient's head rotated 45º in the opposite direction and the microscope tilted 25º toward the surgeon. Under direct gonioscopic visualization, the KDB was inserted into the trabecular meshwork (TM), excising approximately 90º-100º of TM tissue. The blade and viscoelastic were then removed. All procedures were performed by one of three experienced glaucoma specialists.
Follow-up and outcomes
Patients were evaluated preoperatively and at 1, 6, 12, 24, and 36 months postoperatively. The following parameters were assessed: IOP, number of hypotensive eye drops, best-corrected visual acuity, pachymetry, and glaucoma staging.
Surgical success was defined as either an IOP reduction of >20% from baseline with IOP between 6 and 18 mmHg without additional medications or a reduction of ≥1 eye drop from baseline with IOP between 6 and 18 mmHg. Patients who did not meet these criteria or required further IOP-lowering interventions after phaco-KDB were considered failures.
Statistical analysis
Descriptive statistics included minimum, maximum, median, mean, standard deviation, and coefficient of variation for continuous variables, and frequency and percentage for categorical variables. To evaluate the effects on IOP and the number of eye drops, a one-sample proportion test was applied. Kaplan-Meier survival analysis was used to determine the proportion of patients achieving surgical success over time. Complications were recorded by type and frequency.
Statistical analyses were performed using SPSS software (version 23; IBM Corp., Armonk, New York) and LibreOffice Community 7.1.0.3. A p-value ≤0.05 was considered statistically significant.
RESULTS
The medical records of 56 consecutive eyes from 56 patients who underwent phacoemulsification combined with KDB goniotomy (phaco-KDB) between April 2019 and March 2020 were reviewed. All patients had complete follow-up data for 36 months.
The demographic and preoperative characteristics are summarized in table 1. The mean age was 62.9 years; most patients were male (55.4%) and self-identified as mixed race (80.4%). All patients had mild-to-moderate primary open-angle glaucoma. The mean visual field mean deviation (MD) was −4.75 ± 2.08 dB, assessed with the 24-2 SITA Standard strategy on the Humphrey Visual Field Analyzer (HFA 740i, ZEISS Medical Technology). Approximately 36% of patients had MD values worse than −6 dB, meeting the criteria for moderate glaucoma, although the overall mean was within the mild range.
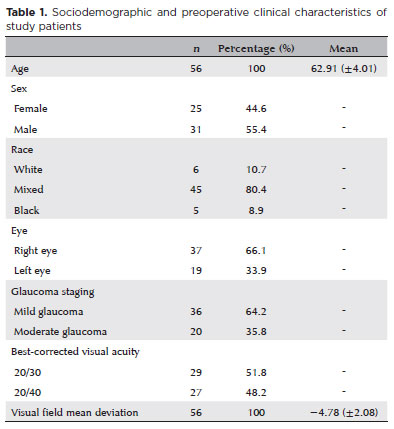
The mean preoperative IOP was 15.96 ± 2.83 mmHg. On the first postoperative day, IOP increased to 17.45 ± 5.78 mmHg. By 1 month, mean IOP decreased significantly to 12.93 ± 3.08 mmHg (p<0.001) and remained consistently reduced throughout follow-up: 12.21 ± 1.95 mmHg at 6 months, 12.41 ± 1.91 mmHg at 12 months, 12.98 ± 1.92 mmHg at 24 months, and 13.14 ± 2.11 mmHg at 36 months (all p<0.001; Table 2). Compared with baseline, all follow-up points showed statistically significant reductions in IOP, with absolute decreases ranging from 3.75 to 2.82 mmHg, corresponding to a 22% to 14% decline.
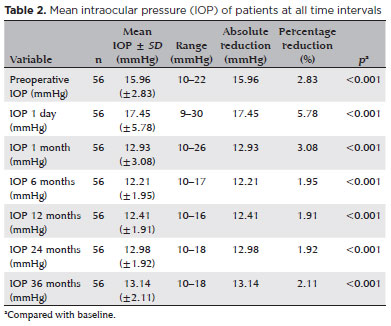
A significant reduction in the number of hypotensive eye drops was also observed. At 1 month, the mean number of drops decreased to 0.95 ± 0.84 (p<0.001), with a gradual increase thereafter: 1.11 ± 0.89 at 6 months, 1.16 ± 0.91 at 12 months, 1.29 ± 0.91 at 24 months, and 1.34 ± 0.92 at 36 months (all p<0.001; Table 3). Compared with baseline, medication use decreased by 56.3% at 1 month and by 32.7% at 36 months, with all reductions statistically significant (p<0.05).
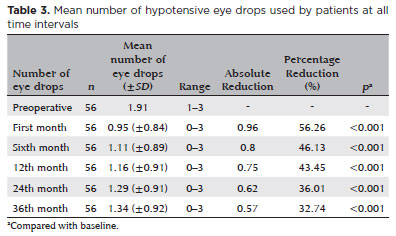
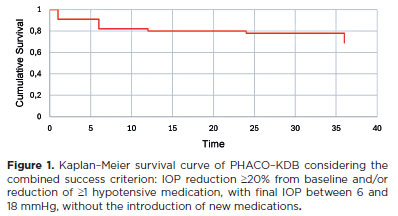
When applying the combined success criterion (IOP reduction ≥20% and/or ≥1 medication reduction, with final IOP between 6 and 18 mmHg), the success rate was 91.1% (n=51) at 1 month (p<0.001), declining gradually to 82.1% at 6 months, 80.4% at 12 months, 78.6% at 24 months, and 69.6% at 36 months (Figure 1). Conversely, failure rates increased to 30.4% at 36 months.
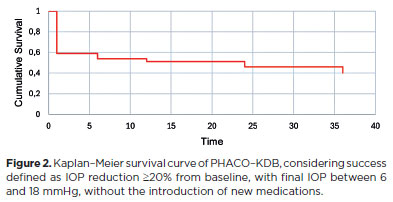
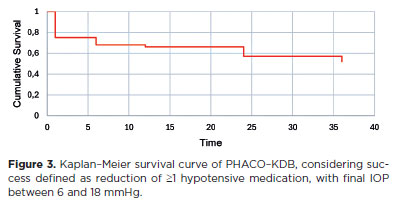
When success was defined strictly as ≥20% IOP reduction from baseline without additional medication and a final IOP between 6 and 18 mmHg, success rates were 58.9%, 53.5%, 51.2%, 46.4%, and 39.3% at 1, 6, 12, 24, and 36 months, respectively (Figure 2). Alternatively, when defined as a reduction of ≥1 medication from baseline with a final IOP between 6 and 18 mmHg, success rates were higher: 75% at 1 month, 67.8% at 6 months, 66.6% at 12 months, 57.1% at 24 months, and 51.8% at 36 months (Figure 3).
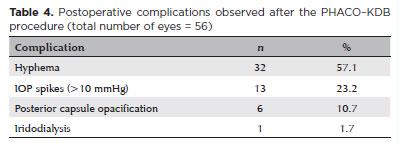
The most common complications included transient IOP spikes (defined as postoperative IOP exceeding baseline by >10 mmHg), hyphema, and posterior capsule opacification. Hyphema occurred in 57.1% of patients during the early postoperative period but was mild in all cases and resolved spontaneously within the first week without medical or surgical intervention. One patient experienced a small intraoperative iris dialysis, which resolved uneventfully without further treatment. No cases of hypotony, malignant glaucoma, choroidal effusion, or other severe complications were reported (Table 4). No patient required reoperation to control IOP. All treatment failures were due to the reintroduction or escalation of hypotensive eye drops.
DISCUSSION
This study evaluated the outcomes of cataract surgery combined with goniotomy using the KDB in patients with primary open-angle glaucoma treated within the public health system. The primary objective was to assess efficacy and safety over a 3-year follow-up.
With respect to IOP reduction, an average decrease of 20% was maintained throughout the first year of follow-up. This result is consistent with previous studies reporting reductions between 11% and 34% when KDB is combined with cataract surgery(10,13,14). At 36 months, the IOP reduction was 14.8%, which is lower than the 34.7%-36.9% reductions described elsewhere(16,17). This difference may be explained by the baseline characteristics of our cohort, in which most patients already had well-controlled IOP (<18 mmHg) and lacked major predictors of large reductions, such as high baseline IOP, short axial length, pseudoexfoliation syndrome, or dense cataracts(18,19). Despite this, the procedure provided consistent IOP-lowering benefits, even in patients with mild-to-moderate, stable disease.
The IOP-lowering effect of cataract surgery alone must also be considered. Phacoemulsification by itself has been shown to reduce IOP modestly-by approximately 1 to 1.4 mmHg after 12 months-and this effect tends to diminish over time(20,21). Although this study did not include a control group undergoing phacoemulsification alone, the greater and sustained IOP reduction observed suggests that phaco-KDB confers additional benefit. Removal of TM by the KDB likely enhances aqueous outflow beyond the effect of cataract surgery alone.
In terms of medication use, a 39.2% reduction was observed in the first year, with a 29.8% reduction sustained at 36 months. These findings fall within the 11%-79% reduction reported in the literature(10,13,14). Although medication use gradually increased over time, the reduction remained statistically significant at all follow-up points. This outcome is clinically meaningful, as reducing the medication burden improves adherence, lowers the risk of side effects, and enhances quality of life.
When surgical success was defined as ≥20% IOP reduction or a decrease of ≥1 hypotensive medication with IOP maintained between 6 and 18 mmHg, success rates were 91.1% at 1 month, 80.4% at 12 months, and 69.6% at 36 months. These results are comparable with outcomes of other minimally invasive glaucoma surgeries (MIGS) targeting the trabecular outflow pathway(19-22). However, success rates were lower when IOP reduction alone was considered, with only 39% of patients achieving ≥20% reduction at 36 months. This likely reflects a ceiling effect, as most patients had low baseline IOP, limiting the extent of possible reduction. Importantly, this should not be interpreted as treatment failure but rather as a function of baseline disease stability.
By contrast, reduction in medication burden was more consistent, particularly in the first year. At 36 months, more than half of patients were still using fewer drops than at baseline. This suggests that, in real-world settings, especially for patients with mild-to-moderate glaucoma, the principal benefit of KDB may be medication-sparing rather than aggressive IOP lowering. This aligns with evolving treatment goals that emphasize quality of life and adherence.
Comparisons with other MIGS procedures must be interpreted cautiously due to differences in study criteria. Gonioscopy-assisted transluminal trabeculotomy combined with cataract surgery has shown greater IOP reduction than phaco-KDB over 12 months (37%-44% vs. 22.9%), although success rates were similar in the first year (87% vs. 80%)(22-24). Compared with the iStent bypass (Glaukos, San Clemente, California), which produces 27%-38% IOP reductions at 12 months, our findings (22.9%) are within a comparable range(25-27). Success rates were also similar (83% iStent vs. 80% KDB)(27). While stent-based procedures minimize trabecular disruption, KDB goniotomy offers a lower-cost, stent-free alternative, avoiding risks such as implant obstruction, migration, or fibrosis. Moreover, by excising a segment of TM, KDB may provide broader access to Schlemm's canal and potentially more durable IOP control.
Postoperative complications were generally mild and self-limited. Hyphema was the most frequent (57.1%), followed by transient IOP spikes (23.2%) and posterior capsule opacification. All hyphema resolved spontaneously within 1 week without intervention. A single case of minor intraoperative iridodialysis was reported and resolved uneventfully. No hypotony, malignant glaucoma, or reoperations were observed.
Transient IOP spikes occurred in 23.2% of cases, typically within the first two postoperative weeks. These elevations, defined as >10 mmHg above baseline, may be related to residual viscoelastic, postoperative inflammation, or temporary outflow resistance at Schlemm's canal(10,14). Although self-limiting, such spikes could pose a risk in patients with advanced optic nerve damage. For this subgroup, close postoperative monitoring and early use of IOP-lowering medications are advisable.
This study has several limitations. The absence of a control group (e.g., phacoemulsification alone) precludes definitive attribution of efficacy to KDB. All procedures were performed by experienced glaucoma specialists in patients with mild-to-moderate disease, which may limit generalizability. Additionally, while surgical success remained significant across follow-up, the survival rate declined from 91.1% at 1 month to 69.6% at 36 months, highlighting the need for continued monitoring and individualized management.
In conclusion, phacoemulsification combined with KDB goniotomy is a safe and moderately effective option for patients with mild-to-moderate primary open-angle glaucoma, particularly in public health settings. While efficacy diminishes gradually, the procedure provides meaningful short- and intermediate-term benefits, primarily by reducing medication burden, with a favorable safety profile.
AUTHORS' CONTRIBUTIONS:
Significant contribution to conception and design: José Paulo Cabral de Vasconcelos; Antônio Lucas Oliveira Correia. Data acquisition: Antônio Lucas Oliveira Correia; Breno Santos Holanda. Data analysis and interpretation: José Paulo Cabral de Vasconcelos; Antônio Lucas Oliveira Correia. Manuscript drafting: Antônio Lucas Oliveira Correia. Significant intellectual content revision of the manuscript: José Paulo Cabral de Vasconcelos; Vital Paulino Costa. Final approval of the submitted manuscript: Antônio Lucas Oliveira Correia; Breno Santos Holanda; Vital Paulino Costa; José Paulo Cabral de Vasconcelos. Statistical analysis: Antônio Lucas Oliveira Correia; José Paulo Cabral de Vasconcelos. Obtaining funding: not applicable. Supervision of administrative, technical, or material support: José Paulo Cabral de Vasconcelos. Research group leadership: Antônio Lucas Oliveira Correia.
REFERENCES
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-7.
2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90.
3. Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-20; discussion 829-30. Comment in: Arch Ophthalmol. 2004;122(7):1088-9, author reply 1089.
4. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429-40. Comment in: Am J Ophthalmol. 2000;130(4):490-1.
5. Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123(1):129-40. Comment in: Ophthalmology. 2016;123(1):e65-66. Ophtalmology. 2016;123(11):e66.
6. Scheie HG. Goniotomy and the treatment of congenital glaucoma. Am J Ophthalmol. 1950;33(6):977-9.
7. Barkan O. Present status of goniotomy. Am J Ophthalmol. 1953;36(4):445-53.
8. Ikeda H, Ishigooka H, Muto T, Tanihara H, Nagata M. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch Ophthalmol. 2004;122(8):1122-8.
9. Seibold LK, SooHoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(3):524-529.e2.
10. Dorairaj S, Radcliffe NM, Grover DS, Brubaker JW, Williamson BK. A review of excisional goniotomy performed with the Kahook Dual Blade for glaucoma management. J Curr Glaucoma Pract. 2022;16(1):59-64.
11. Ansari E, Loganathan D. 12-month clinical outcomes of combined phacoemulsification and ab interno trabeculectomy for open-angle glaucoma in the United Kingdom. PLoS One. 2021;16(6):e0252826.
12. ElMallah MK, Seibold LK, Kahook MY, Williamson BK, Singh IP, Dorairaj SK; KDB Goniotomy Study Group. 12-month retrospective comparison of Kahook Dual Blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv Ther. 2019;36(9):2515-27.
13. Berdahl JP, Gallardo MJ, ElMallah MK, Williamson BK, Kahook MY, Mahootchi A, et al. Six-month outcomes of goniotomy performed with the Kahook Dual Blade as a stand-alone glaucoma procedure. Adv Ther. 2018;35(11):2093-102.
14. Dorairaj SK, Seibold LK, Radcliffe NM, Aref AA, Jimenez-Román J, Lazcano-Gomez GS, et al. 12-month outcomes of goniotomy performed using the Kahook Dual Blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35(9):1460-9.
15. Iwasaki K, Kakimoto H, Orii Y, Arimura S, Takamura Y, Inatani M. Long-term outcomes of a Kahook Dual Blade procedure combined with phacoemulsification in Japanese patients with open-angle glaucoma. J Clin Med. 2022;11(5):1354.
16. Hodapp E, Anderson H, Parrish R, Chang TC, Ramulu P, Hodapp E. Evaluating the asymptomatic patien. In: Chang TC, Ramulu P, Hodapp E editors. Clinical Decisions in Glaucoma. St. Louis: Mosby; 1993. p. 53.
17. Wagner IV, Boopathiraj N, Lentz C, Dorairaj EA, Draper C, Kumar D, et al. Long-term efficacy of successful excisional goniotomy with the kahook dual blade. Clin Ophthalmol. 2024;18:713-21.
18. Kornmann HL, Fellman RL, Feuer WJ, Butler MR, Godfrey DG, Smith OU, et al. Early results of goniotomy with the Kahook Dual Blade, a novel device for the treatment of glaucoma. Clin Ophthalmol. 2019;13:2369-76.
19. Leal I, Chu CJ, Yang YY, Manasses DM, Sebastian RT, Sparrow JM. Intraocular pressure reduction after real-world cataract surgery. J Glaucoma. 2020;29(8):689-93.
20. Zetterström C, Behndig A, Kugelberg M, Montan P, Lundström M. Changes in intraocular pressure after cataract surgery: analysis of the Swedish National Cataract Register Data. J Cataract Refract Surg. 2015;41(8):1725-9.
21. Rahmatnejad K, Pruzan NL, Amanullah S, Shaukat BA, Resende AF, Waisbourd M, et al. Surgical outcomes of gonioscopy-assisted Transluminal Trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma. 2017;26(12):1137-43.
22. Hirabayashi MT, Lee D, King JT, Thomsen S, An JA. Comparison of surgical outcomes of 360º circumferential trabeculotomy versus sectoral excisional goniotomy with The Kahook Dual Blade at 6 months. Clin Ophthalmol. 2019;13:2017-24.
23. Faria BM, Costa VP, Melillo GH, Daga FB, Scoralick AL, Paranhos A Jr, et al. Gonioscopy-assisted transluminal trabeculotomy for glaucoma: 1-year outcomes and success predictors. J Glaucoma. 2022;31(6):443-8.
24. Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407-12.
25. Fea AM, Belda JI, Rękas M, Jünemann A, Chang L, Pablo L, et al. Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875-82.
26. Falkenberry S, Singh IP, Crane CJ, Haider MA, Morgan MG, Grenier CP, et al. Excisional goniotomy vs trabecular microbypass stent implantation: a prospective randomized clinical trial in eyes with mild to moderate open-angle glaucoma. J Cataract Refract Surg. 2020;46(8):1165-71.
Submitted for publication:
May 7, 2025.
Accepted for publication:
August 26, 2025.
Approved by the following research ethics committee: Hospital Geral de Fortaleza/SUS (CAAE: 64265522.1.0000.5040).
Data Availability Statement: The datasets generated and/or analyzed during the study are available from the corresponding author upon reasonable request and can be provided by referees if required.
Edited by
Editor-in-Chief: Newton Kara-Júnior Associate Editor: Heloísa Russ
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: VPC Consultant: Abbvie, Zeiss, Alcon. Lectures: Zeiss, Abbvie, Retinalize, Genom, Glaukos. The other authors declare no potential conflicts of interest.