

Amanda F. L. Morais1; Luisa M. Hopker2; Nilva S. B. Moraes1; Bernardo Reichert3; Murilo V. De Prá3; Anna Carolina B. Linhares4; Ricardo M. Takashima5; Norma Allemann1
DOI: 10.5935/0004-2749.2024-0170
ABSTRACT
PURPOSE: To assess the sensitivity and specificity of the retinopathy of prematurity score (ROPScore) and weight, insulin-like growth factor-1, retinopathy of prematurity algorithm in predicting the risk of developing severe retinopathy of prematurity (prethreshold type 1) in a sample of preterm infants in Brazil.
METHODS: Retrospective analysis of medical records of preterm infants (n=288) with birth weight of ≤1500 g and/or gestational age of 23-32 weeks in a neonatal unit in Southern Brazil from May 2013 to December 2020 (92 months).
RESULTS: The incidence of confirmed severe retinopathy of prematurity was 6.6%. ROPScore showed a 100% sensitivity, 44.6% specificity (95% confidence interval [CI] 38.7-50.6), 11.3% positive predictive value (95% CI 6.5-16.1), and 100% negative predictive value in predicting severe retinopathy of prematurity. The weight, insulin-like growth factor-1, retinopathy of prematurity algorithm demonstrated a 78.9% sensitivity (95% CI 60.6-97.3), 51.3% specificity (95% CI 45.3-57.3), 10.3% positive predictive value (95% CI 5.3-15.2), and 97.2% negative predictive value (95% CI 94.5-99.9).
CONCLUSION: ROPScore identified all patients at risk for severe retinopathy of prematurity. These findings support incorporating ROPScore into Brazilian guidelines to optimize retinopathy of prematurity screening and reduce unnecessary ophthalmologic examinations. Weight, insulin-like growth factor-1, retinopathy of prematurity's suboptimal performance in this Brazilian sample highlights the need for country-specific algorithm adjustments.
Keywords: Retinopathy of prematurity; ROPScore, WINROP; Prediction algorithm; Infant, premature
INTRODUCTION
Retinopathy of prematurity (ROP) has far-reaching consequences, imposing significant financial and social burdens on communities. Beyond the risk of irreversible vision loss, ROP can also lead to cognitive and psychomotor impairments, impacting the long-term development of the affected children(1,2). The current ROP screening process, involving ophthalmological examinations, can be distressing for premature infants(3,4). Furthermore, there is a scarcity of experienced ophthalmologists for ROP screening in both high and low-income countries(5). Therefore, it is imperative to assess the currently available screening algorithms to facilitate the detection of preterm newborns at risk of developing ROP and requiring treatment. This can help optimize the screening protocols, reducing the number of unnecessary examinations for low-risk children(6-8).
The Weight, Insulin-like Growth Factor-1, Retinopathy of Prematurity (WINROP) algorithm, developed in Sweden, is a predictive tool to identify newborns at risk of severe ROP. This online application is designed for newborns with a gestational age (GA) between 23 and 32 weeks. The algorithm functions by comparing the newborn's weight each week with a normalized growth curve for infants who did not develop ROP or who developed mild ROP. Any differences between the expected and actual weights accumulate each week. When these cumulative deviations exceed a predetermined threshold, the system triggers a red alert, signaling the risk of development of severe ROP development in the newborn(9-11).
The Retinopathy of Prematurity Score (ROPScore) algorithm was developed in Brazil to predict severe ROP. It utilizes birth weight (BW), GA, weight gain proportional to body weight at 6 weeks of life, need for blood transfusion, and use of oxygen in mechanical ventilation as predictive variables. The algorithm's creator proposed that ROPScore evaluation can be performed in the 2nd week of life instead of the 6th week, allowing for earlier screening(12). A score of ≥11 indicates a risk of ROP (any stage), while a score of ≥14.5 signals a risk of severe ROP(7). Infants with ROP score of ≥14 require more frequent monitoring owing to the high risk of developing severe ROP.
The primary objective of this study was to evaluate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of ROPScore and WINROP for predicting the risk of developing ROP or severe ROP (prethreshold type 1), as well as the accuracy of these algorithms.
METHODS
Study design and participants
This observational, cross-sectional, retrospective study analyzed data from the neonatal intensive care unit at Hospital do Trabalhador in Brazil, covering a 92-month period from May 2013 to December 2020. The inclusion criteria was newborns with a BW of ≤1,500 kg and/or GA of 23-32 weeks who underwent ROP screening and for whom the necessary medical data for the application of ROPScore and WINROP were available.
Out of the 321 premature infants reviewed, 6 were excluded due to incomplete medical records, and 27 were excluded because the GA exceeded 32 weeks. Therefore, 288 premature infants were included in the analysis.
ROP screening and classification
All premature infants enrolled in this study underwent ophthalmologic examinations performed by a single ophthalmologist between the 4th and 6th week of life. The examinations adhered to the Brazilian guidelines, continuing up to the GA of 45 weeks, until complete retinal vascularization or complete regression of ROP(13). Examination frequency varied, occurring weekly or less frequently, contingent upon the ophthalmological findings. Before the examination, pupils were dilated using three instillations spaced five minutes apart. A drop of 0.5% tropicamide (Mydriacyl 0.5%®, Alcon Laboratórios do Brasil Ltda.) and a drop of 2.5% phenylephrine hydrochloride (Fenilefrina 2.5%®, Allergan Produtos Farmacêuticos Ltda.) were used approximately 40 minutes before the examination. Retinal fundus examination was then performed using a binocular indirect ophthalmoscope and a 28-diopter lens. The premature infants were positioned in dorsal decubitus. A blepharostat was used after the instillation of anesthetic eye drops.
Severe ROP was defined as ROP requiring treatment (type 1 prethreshold ROP), in accordance with the early treatment for retinopathy of prematurity (ETROP) criteria(14).
WINROP algorithm
The algorithm is available online (www.winrop.com). On the website's homepage, a unique identifier was created for each newborn, and their date of birth, estimated due date (GA of 40 weeks), GA, and BW were inputted. Subsequently, the weekly weights of each premature infant, obtained from their electronic medical records, were added. These weekly weights were included until either the algorithm triggered an alarm signal or the infant was discharged. The platform then indicated whether a red alarm signal was triggered, signifying a risk of developing severe ROP, along with the specific week the signal was activated. Subsequently, newborns were divided into two groups based on the presence or absence of the WINROP alarm signal. The online model's performance was then evaluated by calculating the sensitivity (probability of red alarm signal given confirmed severe ROP) and specificity (probability of no red alarm signal given no severe ROP). Using these values, along with the 6.6% prevalence of confirmed severe ROP (19/288), the PPV and NPV were calculated. The PPV indicated the probability of confirmed severe ROP given a positive red alarm signal. The NPV indicated the probability of not having severe ROP given a negative red alarm signal. Additionally, the overall accuracy of the WINROP algorithm was calculated, reflecting the probability of correct predictions.
ROPScore algorithm
The ROPScore algorithm was applied using the smartphone application "ROP SCORE 3" for IOS (PABEX Corporation). The following data were entered into the application: BW, GA, whether a blood transfusion occurred in the first 6 weeks of life, oxygen use through mechanical ventilation in the first 6 weeks of life, and weight at two weeks of life. The application then calculated the ROPScore based on these inputs. The ROP Score's performance was also evaluated by calculating sensitivity (the probability of obtaining an ROP Score of ≥11 or ≥14.5 given that the newborn has confirmed ROP [any stage] or confirmed severe ROP, respectively) and specificity (the probability of scoring below these thresholds [<11 for any ROP stage and <14.5 for severe ROP] when ROP was not confirmed). Finally, the PPV and NPV were calculated for both ROP and severe ROP using the previously determined sensitivities and specificities. These calculations incorporated the study's observed prevalence of confirmed ROP, which was 38.2% (110/288). The PPV indicated the probability of confirmed ROP (any stage) or confirmed severe ROP, given a ROPScore of ≥11 or ≥14.5, respectively. The NPV indicated the probability of not having confirmed ROP (any stage) or severe ROP, given a ROPScore below these thresholds (<11 or <14.5, respectively). Additionally, the accuracy of the ROPScore algorithm was calculated, representing the probability of correct predictions for confirmed ROP (any stage) or severe ROP, using cutoff values of 11 and 14.5, respectively.
Statistical analysis
The data were processed in an Excel® spreadsheet and analyzed using the IBM SPSS Statistics v.28.0 software. Quantitative variables are presented as mean ± standard deviation (SD). The predictive ability of the algorithms was assessed by calculating sensitivity, specificity, and accuracy values. PPV and NPV were also estimated, factoring the prevalence of ROP in the study population. The normality of the distribution of quantitative variables was assessed using the Kolmogorov-Smirnov test. P-values <0.05 were considered indicative of statistical significance.
RESULTS
Clinical characteristics
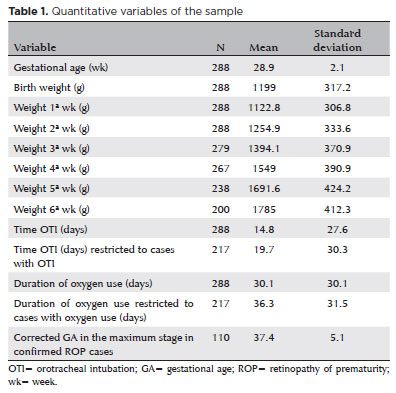
The mean (±SD) GA and BW in the study population were 28.9 ± 2.1 weeks and 1199 ± 317.2 g, respectively. The mean total duration of oxygen use by any means was 30.1 ± 30.1 days. The mean postmenstrual age at the maximum stage of ROP in preterm infants who developed the disease was 37.4 ± 5.1 weeks (Table 1).
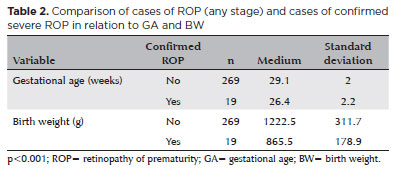
The mean GA and BW of patients who had confirmed severe ROP were lower than those with ROP at any stage, being 26.4 ± 2.2 weeks and 865.5 ± 178.9 g, respectively (Table 2).
ROPScore and WINROP outcomes
The study revealed notable discrepancies between predicted and confirmed severe ROP cases. Notably, 58.3% of patients received a severe ROPScore classification, and 50.7% triggered a positive alarm sign for severe ROP on WINROP. However, ophthalmologic examinations confirmed severe ROP in only 6.6% (n=19) of the study population. Among those with confirmed severe ROP, treatment modalities included laser therapy (9 patients), anti-VEGF Avastin injections (6 patients), and a combination of laser and Avastin treatment (4 patients).
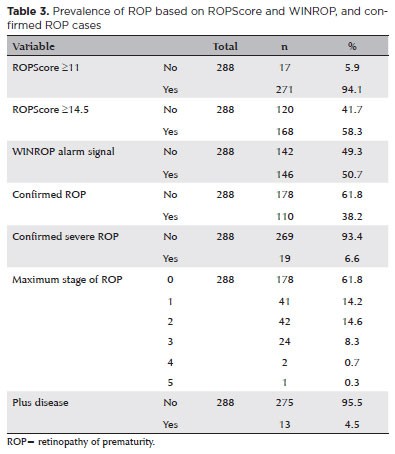
Among the 288 premature infants studied, 61.8% remained free of ROP throughout. The remaining 38.2% developed ROP, with the following distribution: 14.2% had stage 1; 14.6% had stage 2; 8.3% had stage 3; 0.7% had stage 4; and 0.3% had stage 5. Additionally, plus disease was observed in 4.5% of the infants (Table 3).
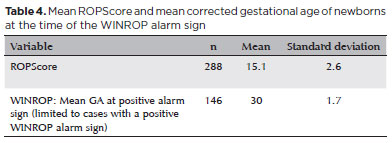
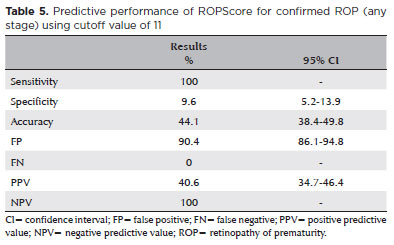
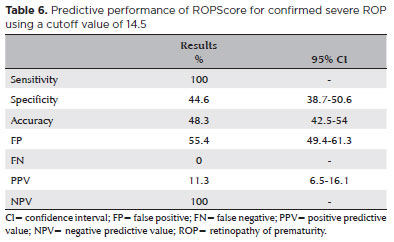
The average ROPScore in this study was 15.1 ± 2.6 points. For WINROP, the mean corrected GA at alarm signal activation was 30 ± 1.7 weeks (Table 4). Notably, the ROPScore showed 100% sensitivity in predicting confirmed ROP (any stage), using a cutoff point of 11 (Table 5). For predicting severe ROP, ROPScore showed a 100% sensitivity, 44.6% specificity (95% confidence interval [CI] 38.7-50.6), 11.3% PPV (95% CI 6.5-16.1), and a 100% NPV (Table 6).
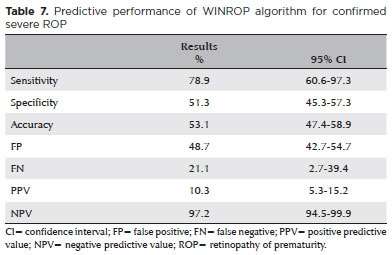
The WINROP algorithm showed a 78.9% sensitivity (95% CI 60.6-97.3), 51.3% specificity (95% CI 45.3-57.3), 10.3% PPV (95% CI 5.3-15.2), and 97.2% NPV (95% CI 94.5-99.9) in predicting severe ROP (Table 7).
DISCUSSION
The current Brazilian guidelines for ROP screening are based solely on GA and BW(13). Consequently, many preterm infants are included in the screening, with all being considered at equivalent risk for severe ROP development. WINROP and ROPScore algorithms offer enhanced risk stratification by incorporating additional variables. This targeted approach enables screening to focus on high-risk infants. An ideal algorithm to identify preterm infants at risk of severe ROP would have a 100% sensitivity with a reasonable level of specificity(15).
Several studies have demonstrated the effectiveness of the WINROP algorithm as a screening tool. However, its sensitivity varies significantly across different countries and economic contexts. In high-income countries such as Sweden, where the algorithm was developed, and the United States of America, WINROP has demonstrated perfect (100%) sensitivity, identifying all preterm infants with severe ROP(9,10). However, middle-income countries such as Mexico have reported lower sensitivity (84%) (16). A potential explanation for this could be that in the Swedish study, no infant with GA >28 weeks developed stage 3 ROP requiring treatment. In developing countries, infants with higher GA are known to develop ROP more often than in developed high-income countries. These findings suggest that screening criteria should be tailored to the specific population and economic context, taking into account local risk factors and disease patterns(1,3).
In the present study, the sensitivity of WINROP (78.9% [95% CI 60.6%-97.3%), was similar to that reported in other middle-income countries such as Mexico. The study identified four premature infants with severe ROP who received treatment, but for whom the algorithm did not trigger an alarm. Notably, these infants had relatively higher GA: 36 weeks, 39 weeks, 43 weeks, and a remarkable 63 weeks. The specificity of the WINROP algorithm in our study was notably lower (51.3%) compared to the original Swedish study (84.5%). This discrepancy resulted in a high rate of false positives and a low PPV (10.3%). Due to this low specificity observed in our study, it would be necessary to generally continue screening for ROP in infants with a positive alarm sign.
The original study that created the ROPScore algorithm obtained a 94% sensitivity and 26% specificity for any stage of ROP. For predicting severe ROP, it showed a 96% sensitivity and 56% specificity. A key advantage of this algorithm lies in its simplicity and practicality, incorporating easily recordable risk factors for ROP, making it suitable for routine use in neonatal intensive care units. Unlike the WINROP algorithm, the ROPScore is recorded only once in a cross-sectional manner(7).
Our study achieved maximum sensitivity in predicting severe ROP, mirroring findings from studies conducted in Brazil and Italy(17,18). Notably, ROPScore showed a 100% NPV for both ROP (any stage) and severe ROP, enabling the secure identification of preterm infants not at risk of developing severe ROP. This can inform a decrease in the frequency of ophthalmologic exams and the inclusion of ROPScore in guidelines for ROP screening.
Given the critical importance of detecting every treatable case of ROP, our findings suggest that the WINROP algorithm lacks sufficient sensitivity for use in this population. Ideally, multicenter prospective studies should evaluate the use of WINROP or the appropriateness of its criteria for the Brazilian population.
ROP screening using artificial intelligence (AI) offers a promising solution to address specialist shortages and potential inconsistencies in diagnosis. However, further development is required to ensure that AI-driven ROP screening meets rigorous standards for fairness, generalizability, and bias control(19).
Potential limitations of this study include its single-center scope and retrospective design. More robust prospective studies can provide more definitive evidence.
To conclude, in this study, ROPScore identified all patients at risk for severe ROP. Our findings support the incorporation of ROPScore into Brazilian guidelines to optimize ROP screening and minimize unnecessary ophthalmologic examinations. The suboptimal performance of WINROP in this Brazilian sample highlights the need for country-specific algorithm adjustments.
AUTHORS' CONTRIBUTIONS:
Significant contribution to conception and design: Amanda Frota Lacerda Morais, Luisa Moreira Hopker, Norma Allemann. Data acquisition: Amanda Frota Lacerda Morais, Bernardo Reichert, Murilo Valandro De Prá, Anna Carolina Badotti Linhares, Ricardo Mokross Takashima. Data analysis and interpretation: Amanda Frota Lacerda Morais, Luisa Moreira Hopker, Nilva Simeren Bueno de Moraes, Norma Allemann. Manuscript manuscript: Amanda Frota Lacerda Morais. Significant intellectual content revision of the manuscript: Amanda Frota Lacerda Morais, Luisa Moreira Hopker, Nilva Simeren Bueno de Moraes, Bernardo Reichert, Murilo Valandro De Prá, Anna Carolina Badotti Linhares, Ricardo Mokross Takashima, Norma Allemann. Final approval of the submitted manuscript: Amanda Frota Lacerda Morais, Luisa Moreira Hopker, Nilva Simeren Bueno de Moraes, Bernardo Reichert, Murilo Valandro De Prá, Anna Carolina Badotti Linhares, Ricardo Mokross Takashima, Norma Allemann. Statistical analysis: Amanda Frota Lacerda Morais, Luisa Moreira Hopker, Norma Allemann. Obtaining funding: not applicable. Supervision of administrative, technical, or material support: Luisa Moreira Hopker. Research group leadership: Luisa Moreira Hopker.
REFERENCES
1. Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5):e518-25.
2. Wheatley CM, Dickinson JL, Mackey DA, Craig JE, Sale MM. Retinopathy of prematurity: recent advances in our understanding. British Journal of Ophthalmology. 2002;86(6):696-700.
3. Hård AL, Löfqvist C, Fortes Filho JB, Procianoy RS, Smith L, Hellström A. Predicting proliferative retinopathy in a Brazilian population of preterm infants with the screening algorithm WINROP. Archives of Ophthalmology. 2010;128(11):1432-6.
4. Belda S, Pallás CR, De la Cruz J, Tejada P. Screening for retinopathy of prematurity: is it painful? Neonatology. 2004;86(3):195-200.
5. Desai S, Athikarisamy SE, Lundgren P, Simmer K, Lam GC. Validation of WINROP (online prediction model) to identify severe retinopathy of prematurity (ROP) in an Australian preterm population: a retrospective study. Eye. 2021;35(5):1334-9.
6. Binenbaum G. Algorithms for the prediction of retinopathy of prematurity based on postnatal weight gain. Clin Perinatol. 2013;40(2):261-70.
7. Eckert GU, Fortes Filho JB, Maia M, Procianoy RS. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond). 2012;26(3):400-6.
8. Lee SK, Normand C, McMillan D, Ohlsson A, Vincer M, Lyons C, Canadian Neonatal Network. Evidence for changing guidelines for routine screening for retinopathy of prematurity. Arch Pediatr Adolesc Med. 2001;155(3):387-95.
9. Hellström A, Hård AL, Engström E, Niklasson A, Andersson E, Smith L, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123(4):e638-45.
10. Löfqvist C, Andersson E, Sigurdsson J, Engström E, Hård AL, Niklasson A et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Archives of Ophthalmology. 2006;124(12):1711-8.
11. Löfqvist C, Hansen-Pupp I, Andersson E, Holm K, Smith LEH, Ley D, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Archives of Ophthalmology. 2009;127(5):622-7.
12. Fortes Filho JB, Eckert G, Tartarella M, Fortes B, Procianoy R. Revisiting the ROPScore: new evidences and recommendations for users. In: 37o SIMASP. São Paulo; 2014.
13. Zin A, Florêncio T, Fortes Filho JB, Nakanami CR, Gianini N, Graziano RM, et al. Proposta de diretrizes brasileiras do exame e tratamento de retinopatia da prematuridade (ROP). Arq Bras Oftalmol. 2007;70(5):875-83.
14. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684-94.
15. Thomas D, Madathil S, Thukral A, Sankar MJ, Chandra P, Agarwal R, et al. Diagnostic accuracy of WINROP, CHOP-ROP and ROPScore in detecting type 1 retinopathy of prematurity. Indian Pediatr. 2021;58(10):915-21.
16. Zepeda-Romero LC, Hård AL, Gomez-Ruiz LM, Gutierrez-Padilla JA, Angulo-Castellanos E, Barrera-de-Leon JC, et al. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Archives of Ophthalmology. 2012;130(6):720-3.
17. Cagliari PZ, Lucas VC, Borba IC, Leandro DMK, Gascho CL, Veras TN, et al. Validation of ROPScore to predict retinopathy of prematurity among very low birth weight preterm infants in a southern Brazilian population. Arq Bras Oftalmol. 2019;82(6):476-80.
18. Piermarocchi S, Bini S, Martini F, Berton M, Lavini A, Gusson E, et al. Predictive algorithms for early detection of retinopathy of prematurity. Acta Ophthalmol. 2017;95(2):158-64.
19. Nakayama LF, Mitchell WG, Ribeiro LZ, Dychiao RG, Phanphruk W, Celi LA, et al. Fairness and generalisability in deep learning of retinopathy of prematurity screening algorithms: a literature review. BMJ Open Ophthalmol. 2023;8(1):e001216.
Submitted for publication:
June 5, 2024.
Accepted for publication:
September 11, 2024.
Approved by the following research ethics committee: Universidade Federal de São Paulo - UNIFESP (CAAE: 50993121.3.1001.5505).
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: The authors declare no potential conflicts of interest.