

Annamaria Ciminelli Barbosa1,2; Maria Clara de Magalhães-Barbosa1,2; Giovanni Nicola Umberto Italiano Colombini2; Arnaldo Prata-Barbosa1,3
DOI: 10.5935/0004-2749.2023-0073
ABSTRACT
PURPOSE: To describe the epidemiological and clinical profile of hospitalized patients with retinoblastoma in Brazil.
METHODS: Using data from the Hospital Cancer Registry of the , patients with the morphological codes of retinoblastoma who were diagnosed between 2000 to 2018, aged 0–19 years, and followed up in registered hospitals (analytical cases) were selected. The relative and absolute frequencies of demographic, clinical, diagnostic, therapeutic, and outcome variables were described. Hospital performance indicators were calculated and compared between hospitals qualified and not qualified to treat pediatric oncology cases and between hospitals with different case volumes (<20, 20–75, >75 cases).
RESULTS: Of the 2,269 identified analytical cases from 86 institutions, 48% were from the Southeast, 54% were male, and 66% were aged <4 years. The proportion of missing data (NA) was too high for several variables. Approximately 84% of the patients were from the public health system, 40% had a positive family history, and 88% had unilateral involvement. The first treatment included surgery in 58.3% of the patients (NA=2), Approximately 36.6% of these patients achieved complete remission, 10.8% achieved partial remission, and 12.7% died (NA=59%). Hospital performance indicators were within the target in >90% of the patients. The median time between the first appointment and diagnosis (6 days, interquartile range [IQR] 1–14) was significantly lower and the median time to death was longer (343 days, IQR, 212-539) in high-volume hospitals (>75 cases) than in medium- and low-volume hospitals.
CONCLUSIONS: Despite the high proportion of missing data, we found that the delay in diagnosis is due to prehospital factors. Additionally, there is a need for educational programs for healthcare professionals and families that emphasize early identification and referral to specialized centers. Future studies should focus on the impact of Hospital Cancer Registry data completeness on outcomes, causes of delay in diagnosis, regional inequalities, and barriers to accessing specialized services.
Keywords: Retinoblastoma/diagnosis; Retinoblastoma/epidemiology; Patient care; Humans; Children; Adolescents; Brazil.
INTRODUCTION
Retinoblastoma is the most common malignant ocular tumor seen in children. It arises due to the inactivation of both alleles of the RB1 tumor suppressor gene (chromosome 13q), resulting in the formation of a defective protein called pRB, which causes cell cycle alterations and disordered cell proliferation(1). The most frequently observed clinical findings are leukocoria, strabismus, and low vision, which may have a unilateral or bilateral presentation. Inflammatory signs such as hypopyon, ocular hyperemia, and even proptosis may appear(2). It mainly affects children aged 0–4 years, with no preference for sex or race. In this age group, in high-income countries, the incidence ranges from 10 to 12.1/1,000,000 children or approximately 1/17,000 live births(3-6). Data are poor or less consistent in low- and middle-income countries, with reportedly lower incidence rates, such as 4.7 in Mexico(7), 5.3 in Pakistan(8), 7.13 in Brazil(9), 7.62 in Lebanon(10), and 7.7 in South Africa(11), per 1,000,000 children in the same age group.
Improvements in diagnostic and therapeutic methods have resulted in higher patient survival rates, especially in high-income countries (almost 99%). There are numerous therapeutic options today, such as intraarterial chemotherapy, brachytherapy, and laser photocoagulation. Together, these therapies can preserve the eyeball and life(12). However, retinoblastoma can still be fatal and associated with a poor prognosis if it is diagnosed late and not treated adequately. Low- and middle-income countries still have high rates of eyeball enucleation and mortality, which are primarily related to late diagnosis of retinoblastoma in advanced and metastatic stages(13).
Worldwide, numerous health policies aim to monitor patients with retinoblastoma. In Brazil, the main database is the Registro Hospitalar de Cancer (RHC) of the Instituto Nacional de Câncer (INCA). Epidemiological studies in the Brazilian population have mainly been conducted in individual institutions; a few have been conducted to assess the quality of care in the country(13,14). In this study, we aimed to describe the epidemiological, clinical profile, and hospital quality of care indicators of patients diagnosed with retinoblastoma between 2000 and 2018 who were included in Brazil’s RHC. We hope that the study findings will contribute to elucidating the care profile for patients with retinoblastoma, identifying difficulties in the line of care, and understanding the impact of the disease in Brazil. This may help further develop effective public health policies to improve patient care.
METHODS
Design, population, and period of study
This was a descriptive retrospective study of hospitalized patients aged 0-19 years who were diagnosed with retinoblastoma from 2000 to 2018 in Brazil and were included in the RHC/INCA database.
Characteristics of the RHC
The RHC/INCA is a national comprehensive database that collects information regarding the diagnosis, treatment, epidemiological profile, and evolution of malignant neoplasms. Hospitals qualified for cancer care by the Brazilian Sistema Único de Saúde (SUS) are required to send data to the RHC. According to the 2010 RHC Manual, the percentage of unregistered patients is insignificant and corresponds to those being treated at private health centers, from which data sharing is optional. The system uses the International Classification of Diseases (ICD) - Tenth Revision (ICD-10), ICD for Oncology - second and third edition (ICD-O2 and ICD-O3), Classification of Malignant Tumors - sixth edition (TNM), International Classification of Childhood Cancer, Classification for Tumors in Adolescents and Young Adults (CAAJ), and the identification codes of Brazilian municipalities from the Instituto Brasileiro de Geografia e Estatística.
Data collection
Data were obtained on July 2021 (https://www.inca.gov.br/numeros-de-cancer/registros-hospitalares-de-cancer-rhc), using the following filters: age, 0–19 years; ICD-10 code, C69.2 for retinoblastoma; first diagnosis, 2000–2018; and ICD-O2 and ICD-O3 codes for histological type, 9510/3 (retinoblastoma, NOS - not otherwise specified), 9511/03 (differentiated retinoblastoma), 9512/3 (undifferentiated retinoblastoma), and 9513/3 (diffuse retinoblastoma).
Data analysis
For demographic, clinical profile, and care features, only the following analytical cases (as described in the RHC Manual) were included: a) patients diagnosed (or not) in the hospital who completed their treatment and were followed up at the RHC hospital; b) patients diagnosed at the RHC hospital whose treatment was initiated at another hospital (recommended as per the plan of the RHC hospital doctors), but who returned to the RHC hospital for treatment completion and follow-up; and c) patients diagnosed at another hospital where specific antineoplastic treatment was started, but who completed their treatment and were followed up at the RHC hospitale.
Performance indicators, such as the process, productivity and quality indicators, were evaluated. The process indicators (volume of care in RHC hospitals and information quality) included the following: number of new cases registered in the period; percentage of analytical cases; percentage of patients who started the diagnosis and treatment process at the hospital; percentage of patients who arrived at the hospital with advanced disease and without a diagnosis; and percentage of patients without information on certain variables such as clinical condition at the beginning of treatment, previous diagnosis and treatment, most important diagnostic bases, staging, main reason for not undergoing the first treatment at the hospital, first treatment received, and disease status at the end of the first treatment. The productivity indicators, which are related to the hospital’s efficiency in treating patients, were estimated in patients who started the diagnosis and treatment process at the RHC hospital. They included the median time between (i) screening and the first appointment at the clinic that administered the treatment, (ii) first appointment and the first confirmed diagnosis, (iii) first confirmed diagnosis and the beginning of the first antineoplastic treatment, (iv) screening and the initiation of the first anticancer treatment, and (v) first confirmed diagnosis and death. The quality indicators, which are related to the effectiveness of care at the RHC hospital, included the following: percentage of analytical cases without evidence of the disease at the end of the first antineoplastic treatment and death rate in the first year after diagnosis.
The median time intervals between first appointment and the first confirmed diagnosis, first confirmed diagnosis and the beginning of the first antineoplastic treatment, and (v) first confirmed diagnosis and death were estimated in hospitals qualified and not qualified for treating pediatric oncology cases and compared using the Mann–Whitney test. These intervals were also estimated and compared using the Kruskal–Wallis test, according to the volume of cases treated in each hospital (<20, 20 to 75, and >75). The significance level was set at 0.05. All analyses were performed using GraphPad (version 9.5.1; Dotmatics, Boston, MA, USA).
RESULTS
From 2000 to 2018, 2,821 patients with retinoblastoma from 104 hospitals were registered in the RHC. Of these, 2,269 (80%) patients from 86 hospitals were classified as analytical cases, which included 1,084 (47.8%) patients from 37 hospitals (43.0%) in the Southeast, 604 (26.7%) patients from 20 hospitals (23.3%) in the Northeast, 252 (11.2%) patients from 16 hospitals (18.6%) in the South, 182 (8.0%) patients from eight hospitals (9.3%) in the North, and 142 (6.3%) patients from five hospitals (5.8%) in the Midwest.
Several demographic, clinical, diagnostic, and therapeutic variables had significant proportions of missing data. According to the available data, 54% of the patients were male, 66% were aged 1–4 years, 50% were brown, 84% were from SUS-approved hospitals, 40% had a positive family history, 54% had not been previously diagnosed or undergone treatment, 88% had unilateral ocular involvement, and 91% had only one tumor at the time of diagnosis (Tables 1 and 2). The staging data were too inconsistent to estimate its distribution.
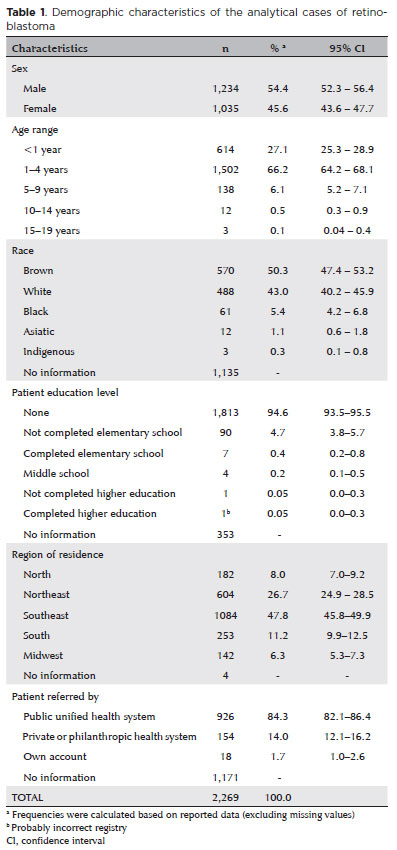
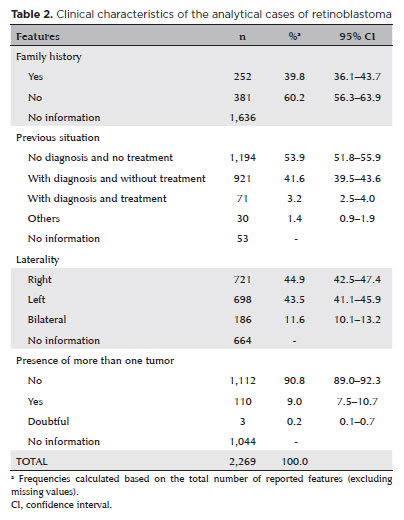
Pathological examination was the leading test for diagnosing retinoblastoma (70.7%), and the primary tumor histology was the most important basis for the diagnosis (68%). However, both variables had >50% missing data. The first treatment (only two missing values) was chemotherapy (74.5%) or surgery (58.3%, no reported type), and they were usually associated with other therapies. At the end of the first treatment at the hospital, 36.6% of the patients achieved complete remission, 10.8% achieved partial remission, and 12.7% died during the study period (59% of the values were missing). Deaths were computed when the “date of death” cell was filled in (303 patients, 13.4%). Empty cells were considered undead (Table 3).
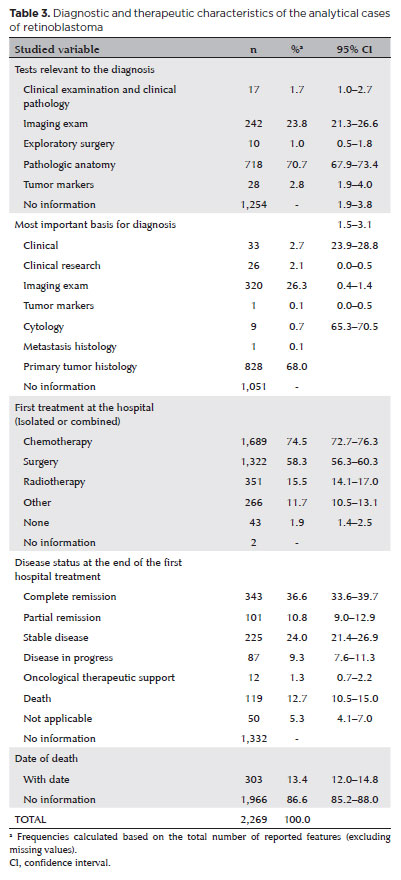
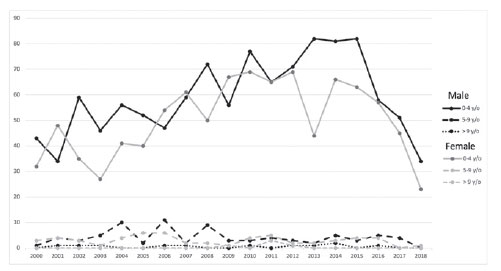
Until 2014, there was an increase in the number of patients screened (37%), diagnosed (33%), who attended their first appointment (33%), in whom specific treatment was initiated (33%), and who died (28.4%) (Table 4). The annual distribution of the analytical cases according to sex and age are shown in figure 1. Approximately 54% of the analytical cases began the diagnosis and treatment process at a registered hospital. We could not estimate the proportion of patients who arrived with an advanced disease or without a diagnosis. The median time between screening and the first appointment, first appointment and the confirmed diagnosis, confirmed diagnosis and the beginning of the first antineoplastic treatment, and screening and the initiation of the first anticancer treatment were within the RHC goals in >90% of the reported cases (50% of screening data were missing). The mortality rate of the 1,194 cases that presented without a diagnosis or having undergone any treatment was 13.2%; approximately, 61.4% of these deaths occurred in the first year of diagnosis (Table 5).
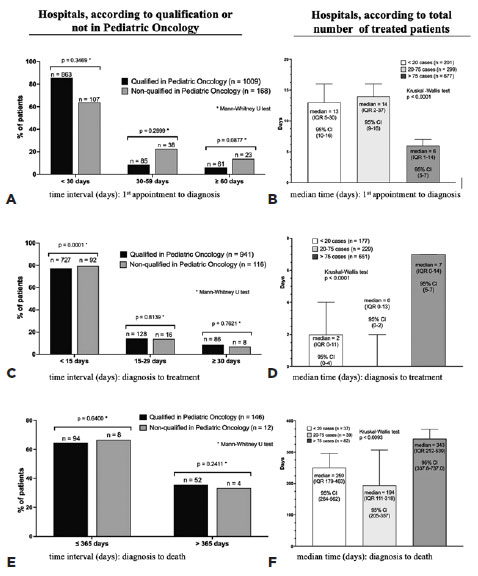
The percentage of cases treated in hospitals qualified for pediatric oncology (QPO) varied among regions: South, 97.5%; Southeast, 95.6%; North, 89.9%; Northeast, 72.3%; and Midwest, 48.2%. Although the percentage of patients with <30 days between the first consultation and diagnosis was higher in QPO hospitals than in non-QPO hospitals, the difference was not significant (p=0.3469, Figure 2A). However, hospitals with high volume of care (>75 cases) demonstrated a significantly shorter median time interval (6 days, interquartile range [IQR] 1-14) than those with medium and low volume of care (p<0.001, Figure 2B). QPO hospitals had a significantly lower percentage of patients (p=0.0001, Figure 2C) than non-QPO hospitals in whom treatment was initiated <15 days after diagnosis. This median time interval was significantly longer (7 days, IQR 0–14) in high-volume hospitals than medium- and low-volume hospitals (p<0.0001, Figure 2D). However, 75% of patients in all the hospitals had an interval of <15 days. No difference was observed between QPO hospitals or in the proportion of patients who died (Figure 2E). However, median survival was longer in high-volume hospitals (343 days, IQR 212–539) than in in medium- and low-volume hospitals (p<0.0093, Figure 2F).
DISCUSSION
The present study, which used data from the RHC/INCA in Brazil, demonstrated that most cases of retinoblastoma occurred in the age group of 1-4 years, with a slight predominance in males. We also found high proportions of missing values in several variables. Most reported cases had unilateral involvement and no significant family history. Pathological anatomy was primarily used to confirm the diagnosis. In most patients, treatment included chemotherapy and surgery. The mortality rate was high, and most deaths occurred in the first year of diagnosis. Time-dependent indicators were within the target established in >90% of the patients, which suggests that the quality of in-hospital care is good.
The probable cause of missing data was failure to complete the RHC or medical record. This suggests that the data may be missing at random, resulting in similar distributions of variables for the reported and missing data. However, it is essential to host regular training programs to raise awareness about the importance of correctly filling out data to generate accurate information.
The demographic and clinical features of this study’s population are in accordance with those of several worldwide studies(6,15). Most patients were from the Southeast region, which is the wealthiest, most populated, and most developed region, with the most significant number of oncology hospitals. However, it is also possible that this region received many referred patients from less developed areas. However, previous studies have reported the highest incidence of retinoblastoma from the Northeast region (e.g., Natal and Salvador), which is socioeconomically less favored(9). Some studies have identified an association between retinoblastoma prevalence and unfavorable environmental risk factors, such as coinfection with HPV(16), maternal diet, and low folate intake(17).
In this study, inconsistent data on tumor staging prevented its reliable estimation. Other studies have demonstrated high percentages of advanced-stage diagnosis in the Brazilian population(18-21). Chantada et al.(22) demonstrated that patients with familial retinoblastoma from developing countries (Argentina, Brazil, Jordan, Turkey, and Venezuela) were diagnosed significantly later and with a more advanced intraocular disease and an increased risk for bilateral enucleation than patients from the USA (only 25% of patients presented with stage 5 retinoblastoma). This reflects a delay in diagnosis, which may be multifactorial. Mattosinho et al.(13) demonstrated that maternal education and time between the first consultation and referral were significantly related to advanced-stage diagnosis and survival in patients with retinoblastoma presenting to INCA, Rio de Janeiro. The time between the first symptoms and referral to an oncology center accounts for an alarming percentage (70%) of the overall gap between diagnosis and treatment in Brazil, compared with 23% in other developing countries(23). These findings suggest that primary care physicians in the Brazilian health system need to be made more aware of retinoblastoma. Therefore, health programs should emphasize awareness among family members and medical professionals of the signs and symptoms of retinoblastoma to allow for early diagnosis and a better prognosis.
Pathological anatomy and primary tumor histology were the main diagnostic methods in this study. Currently, retinoblastoma is clinically diagnosed using fundoscopy. Ocular ultrasonography and nuclear magnetic resonance imaging can be requested for tumor staging(24). Histological examination of the tumor is reserved for patients in whom enucleation is necessary, such as those with a more severe disease and with a worse prognosis. Although our findings might suggest a delay in diagnosis, this conclusion could not be made because data were missing in approximately half of the patients. However, data regarding the first hospital treatment had only two missing values. Although there were no data on the type of surgery performed (enucleation, exenteration, or others), the percentage of surgeries performed (58.3%) was lower than the percentage of pathological anatomy (70.7%) or primary tumor histology (68.0%) data, which are diagnostic procedures that require a surgical specimen. This suggests that these pathologic diagnostic variables were overestimated. The percentage of surgeries performed in the present study is approximately the 3-year enucleation rate in high-income countries, according to a recent report (149 countries). The report revealed that the 3-year enucleation rates are much higher in low-income countries (reaching 73.6%) than in high-income countries (59.7%)(25). This may reflect the lack of access to the most modern therapeutic technologies and the diagnosis of advanced-stage diseases in low-income countries(25,26). With the availability of numerous less aggressive treatment options such as intraarterial chemotherapy, thermotherapy, and laser therapy, enucleation surgery is being performed less frequently and is reserved only for severe cases(27,28).
The death rate in this study should be interpreted with caution. When the “date of death” was missing, it was impossible to rule out death. However, the overall case fatality rate was 13.4% among the analytical cases of retinoblastoma, which is high compared with that in high-income countries (0.8–1.0%)(25). In the USA, the overall 5-year survival rate has demonstrated an upward trend, rising from 90.8% in the 1980s to 92.5% in the 1990s and 97.6% in the 2000s(4). The case fatality rate reported in a few studies in Brazil has demonstrated a downward trend. It was 70% from 1956 to 1973 in Rio de Janeiro(29), around 26% from 1975 to 1997 in Recife(30), and 13% from 2006 to 2013 in Rio de Janeiro(13). Among the analytical cases that arrived at the referral center without a diagnosis or having received any treatment, we found a case fatality rate of 13.2%. More than 60% of the deaths occurred in the first year, suggesting advanced-stage disease at diagnosis.
In this study, we observed an increase in the number of hospitalized cases of retinoblastoma until 2014, followed by a decrease in the number. This finding suggests a delay in updating the data in the RHC, which presents an opportunity for improvement. Continuous training in updating the RHC is essential to minimize failures in data collection and obtain a more reliable epidemiological profile of retinoblastoma.
The time-dependent indicators were quite positive, suggesting the good quality of in-hospital care. Most cases of retinoblastoma were treated in QPO hospitals, especially in the South and Southeast regions. In addition, although not significantly different, the percentage of patients with an optimal time interval between the first appointment and diagnosis was higher in QPO hospitals than in non-QPO hospital. Furthermore, this interval was significantly shorter in high-volume hospitals (>75 cases) than in medium- and low-volume hospitals. These findings suggest that the main component of the possible delay in diagnosis is the delay in referral to the tertiary center. The time interval between diagnosis and treatment was within the clinically acceptable range of 15 days in approximately 75% of the patients in all the hospitals. Public health campaigns should focus on raising awareness among family members and primary care professionals regarding the early signs of retinoblastoma and the need for prompt referral of patients to tertiary care centers to improve prognosis.
This study has some limitations inherent to the use of secondary data. Important variables had inconsistent information or no information in a large volume of cases, which could have introduced participation bias in addition to information bias. However, considering that the probable cause of missing data was failure to fill the RHC or medical records, there may be no difference in the characteristics of patients with missing data and those with complete data. However, this could not be demonstrated. Other variables associated with the stage at the time of diagnosis, such as maternal education, were not available. The date of first symptoms was also lacking and hampered the assessment of prediagnostic intervals, another variable associated with prognosis. In addition, the case fatality rate needs to be interpreted with caution because information regarding death depended on the “date of death” data. No casualty was considered when the field was empty, which could have resulted in an underestimation of the fatality rate.
Despite the high proportions of unreported data on retinoblastoma in the Brazilian RHC, the findings of the present study, such as the high mortality rates and positive hospital performance indicators, suggest that patients are presenting to specialized centers with advanced diseases and that the delay in diagnosis is related to prehospital factors. The study results highlight opportunities for the improvement on several fronts, such as incentives and ongoing training programs to fill out and regularly update RHC data, educational programs for primary care physicians and public health campaigns for families regarding the early signs of retinoblastoma, and policies to facilitate access to specialized centers. Future studies evaluating the impact of improving the completeness of RHC data on health outcomes, determining the causes of late diagnosis, assessing regional differences in retinoblastoma treatment, and determining the barriers to accessing specialized services could contribute to the development of strategies to modify the current situation and improve the disease prognosis in Brazil.
ACKNOWLEDGMENTS
This study was supported by the Department of Pediatrics of the D’Or Institute for Research and Education (IDOR).
We thank Dr. Marianna de Camargo Cancela from the Division of Surveillance and Situation Analysis of the National Institute of Cancer (INCA), Ministry of Health, Rio de Janeiro, Brazil, for her kind collaboration and enriching suggestions.
REFERENCES
1. Cruz-Galvez CC, Ordaz-Favila JC, Villar-Calvo VM, Cancino-Marentes ME, Bosch-Canto V. Retinoblastoma: review and new insights. Front Oncol. 2022;12:963780.
2. Dimaras H, Corson TW. Retinoblastoma, the visible CNS tumor: A review. J Neurosci Res. 2019;97(1):29-44.
3. MacCarthy A, Birch JM, Draper GJ, Hungerford JL, Kingston JE, Kroll ME, et al. Retinoblastoma in Great Britain 1963-2002. Br J Ophthalmol. 2009;93(1):33-7.
4. Fernandes AG, Pollock BD, Rabito FA. Retinoblastoma in the United States: a 40-year incidence and survival analysis. J Pediatr Ophthalmol Strabismus. 2018;55(3):182-8.
5. Park SJ, Woo SJ, Park KH. Incidence of retinoblastoma and survival rate of retinoblastoma patients in Korea using the Korean National Cencer Registry Database (1993-2010). Invest Ophthalmol Vis Sci. 2014;55(5):2816-21.
6. Moll AC, Kuik DJ, Bouter LM, Den Otter W, Bezemer PD, Koten JW, et al. Incidence and survival of retinoblastoma in the Netherlands: a register based study 1862-1995. Br J Ophthalmol. 1997; 81(7):559-62.
7. Amozorrutia-Alegria V, Bravo-Ortiz JC, Vazquez-Viveros J, Campos-Campos L, Mejia-Arangure M, Juarez-Ocana S, et al. Epidemiological characteristics of retinoblastoma in children attending the Mexican Social Security Institute in Mexico City, 1990-94. Paediatr Perinat Epidemiol. 2002;16(4):370-4.
8. Bhurgri Y, Muzaffar S, Ahmed R, Ahmed N, Bhurgri H, Usman A, et al. Retinoblastoma in Karachi, Pakistan. Asian Pac J Cancer Prev. 2004;5(2):159-63.
9. Barbosa AC, de Magalhaes-Barbosa MC, Moreira JP, Colombini G, Prata-Barbosa A. Incidence of retinoblastoma in children and adolescents in Brazil: A population-based study. Front Pediatr. 2022;10:1048792.
10. El Hage S, Wakim E, Daou L, El Masri J, Salameh P. Epidemiology and incidence of retinoblastoma in the Middle East: a Nationwide Study in Lebanon. Cureus. 2021;13(10):e18696.
11. Stuart KV, Shepherd DJ, Kruger M, Singh E. The Incidence of retinoblastoma in South Africa: findings from the South African National Cancer Registry (2004–2018). Ophthalmic Epidemiol. 2022;29(6):681-7.
12. Schaiquevich P, Francis JH, Cancela MB, Carcaboso AM, Chantada GL, Abramson DH. Treatment of retinoblastoma: what is the latest and what is the future. Front Oncol. 2022;12:822330.
13. Mattosinho CC, Grigorovski N, Lucena E, Ferman S, Soares de Moura AT, Portes AF. Prediagnostic Intervals in retinoblastoma: experience at an Oncology Center in Brazil. J Glob Oncol. 2017; 3(4):323-30.
14. Mattosinho CC, Moura A, Oigman G, Ferman SE, Grigorovski N. Time to diagnosis of retinoblastoma in Latin America: A systematic review. Pediatr Hematol Oncol. 2019;36(2):55-72.
15. Andreoli MT, Chau FY, Shapiro MJ, Leiderman YI. Epidemiological trends in 1452 cases of retinoblastoma from the Surveillance, Epidemiology, and End Results (SEER) registry. Can J Ophthalmol. 2017;52(6):592-8.
16. Orjuela M, Castaneda VP, Ridaura C, Lecona E, Leal C, Abramson DH, et al. Presence of human papilloma virus in tumor tissue from children with retinoblastoma: an alternative mechanism for tumor development. Clin Cancer Res. 2000;6(10):4010-6.
17. Orjuela MA, Titievsky L, Liu X, Ramirez-Ortiz M, Ponce-Castaneda V, Lecona E, et al. Fruit and vegetable intake during pregnancy and risk for development of sporadic retinoblastoma. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1433-40.
18. Antoneli CB, Steinhorst F, Ribeiro Kde C, Chojniak MM, Novaes PE, Arias V, et al. [The Pediatrician’s ability to recognize the presenting signs and symptoms of retinoblastoma]. Rev Assoc Med Bras (1992). 2004;50(4):400-2. Portuguese.
19. Palazzi MA, Stephan C, Brandalise SR, Aguiar S dos S. Retinoblastoma diagnosis: a proposal based on the experience of Centro Infantil Boldrini, Brazil. Pediatr Hematol Oncol. 2013;30(5):379-85.
20. Selistre SG, Maestri MK, Santos-Silva P, Schuler-Faccini L, Guimaraes LS, Giacomazzi J, et al. Retinoblastoma in a pediatric oncology reference center in Southern Brazil. BMC Pediatr. 2016;16:48.
21. Bonanomi MT, Almeida MT, Cristofani LM, Odone Filho V. Retinoblastoma: a three-year-study at a Brazilian medical school hospital. Clinics (Sao Paulo). 2009;64(5):427-34.
22. Chantada GL, Dunkel IJ, Qaddoumi I, Antoneli CB, Totah A, Canturk S, et al. Familial retinoblastoma in developing countries. Pediatr Blood Cancer. 2009;53(3):338-42.
23. Brasme JF, Morfouace M, Grill J, Martinot A, Amalberti R, Bons-Letouzey C, et al. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. Lancet Oncol. 2012;13(10):e445-59.
24. Jenkinson H. Retinoblastoma: diagnosis and management--the UK perspective. Arch Dis Child. 2015;100(11):1070-5.
25. Global Retinoblastoma Study Group. The Global Retinoblastoma Outcome Study: a prospective, cluster-based analysis of 4064 patients from 149 countries. Lancet Glob Health. 2022;10(8):e1128-e40.
26. Singh G, Daniels AB. Disparities in retinoblastoma presentation, treatment, and outcomes in developed and less-developed countries. Semin Ophthalmol. 2016;31(4):310-6.
27. Shields CL, Shields JA. Diagnosis and management of retinoblastoma. Cancer Control. 2004;11(5):317-27.
28. Temming P, Eggert A, Bornfeld N, Sauerwein W, Goricke S, Lohmann DR. [Diagnosis and treatment of retinoblastoma: current strategies for effective tumour control and preservation of vision]. Klin Monbl Augenheilkd. 2013;230(3):232-42. German.
29. Pawlak BR. Retinoblastoma: an epidemiological study (survey and review). J Surg Oncol. 1975;7(1):45-55
30. Rocha FJ, Erwenne CM, Saba LB, Pacheco JCG. Retinoblastoma: encaminhamento ao Hospital A.C. Camargo/Fundação Antônio Prudente durante 15 anos sequenciais. Arq Bras Oftalmol. 1992; 55(1):7-1.
Submitted for publication:
March 13, 2023.
Accepted for publication:
December 13, 2023.
Approved by the following research ethics committee: Instituto D’Or de Pesquisa e Ensino (CAAE: 59872322.8.0000.5249).
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.