

Marina Viegas Moura Rezende Ribeiro1; Fabiano Timbó Barbosa2; Luiz Eduardo Feliciano Ribeiro3; Célio Fernando de Sousa-Rodrigues4; Eurica Adélia Nogueira Ribeiro5
DOI: 10.5935/0004-2749.20190097
ABSTRACT
This systematic review aimed to assess the effectiveness of using preservative-free artificial tears versus preserved lubricants for the treatment of dry eyes in Universidade Federal de Alagoas (PROSPERO 2018 CRD42018089933). Online databases were searched (LILACS, EMBASE, MEDLINE, and CENTRAL) from inception to April 2018; references from included papers were also searched. The following keywords were used: lubricants OR artificial tears OR artificial tears, lubricants AND dry eye OR dry eye syndrome OR syndromes, dry eye OR dry eyes. Among the 2028 electronic search results, 29 full papers were retrieved and four were considered relevant. The number of participants from these studies ranged from 15 to 76. Meta-analysis was possible for the following outcomes: score of symptoms according to the Ocular Surface Disease Index - Allergan (OSDI), tear secretion rate using the Schirmer test, tear evaporation rate using the tear film breakup time test, burning, foreign body sensation, and photophobia. No statistically significant difference was observed between the two groups, and no side effects were attributed to the interventions. Evidence proving that preservative-free artificial tears are more effective than preserved artificial tears is lacking.
Keywords: Dry eye syndromes/drug therapy; Lubricant eye drops/therapeutic use; Tears; Systematic review
RESUMO
Esta revisão sistemática teve como objetivo avaliar a eficácia do uso de lágrimas artificiais sem conservantes em comparação com lubrificantes preservados no tratamento do olho seco na Universidade Federal de Alagoas (PROSPERO 2018 CRD42018089933). As bases de dados online foram pesquisadas (LILACS, EMBASE, MEDLINE e CENTRAL) desde o início até abril de 2018; referências de artigos incluídos também foram pesquisadas. Foram utilizados os seguintes descritores: lubrificantes OU lágrimas artificiais OU lágrimas artificiais, lubrificantes E olho seco OU síndrome do olho seco OU síndromes, olho seco OU olhos secos. Dos 2028 resultados de busca eletrônica, 29 artigos completos foram recuperados, e quatro foram considerados relevantes. O número de participantes desses estudos variou de 15 e 76. A meta-análise foi possível para as seguintes variáveis: escore de desfecho dos sintomas de acordo com o Índice de Doença da Superfície Ocular - Allergan (OSDI), taxa de secreção lacrimal pelo teste de Schirmer, taxa de evaporação lacrimal usando o teste de tempo de ruptura do filme lacrimal, queimação, sensação de corpo estranho e fotofobia. Nenhuma diferença estatisticamente significativa foi observada entre os dois grupos, e nenhum efeito adverso foi atribuído às intervenções. Evidências provando que as lágrimas artificiais sem conservantes são mais eficazes do que as lágrimas artificiais preservadas estão faltando.
Descritores: Síndromes do olho seco/tratamento farmacológico; Lubrificantes oftálmicos/uso terapêutico; Lágrimas; Revisão sistemática
INTRODUCTION
Based on a recent definition, dry eye syndrome is a multifactorial ocular surface disease characterized by loss of tear film homeostasis, accompanied by ocular symptoms. Its etiology comprises tear film instability, hyperosmolarity, inflammation and ocular surface damage, and neurosensorial alterations. Its prevalence ranges from 5% to 50%(1).
Dry eye disease leads to several ocular symptoms, such as burning, foreign body sensation, blurred vision, and redness, which cause discomfort and reduce working efficiency and quality of life. These symptoms may cause complications, such as corneal damage and visual impairment(2).
Several methods were available for the treatment of dry eyes, such as artificial tears, gels, topical or oral secretagogues, blood derivatives, anti-inflammatory therapy, and punctal occlusion, among others(3).
Artificial tears are the first choice of therapy in dry eye disease(2,4). These drugs enhance tear stability, thus reducing loss by evaporation and inflammation(5). However, this ocular inflammation can be exacerbated with lubricant preservatives, which are also important to prevent microbial activity and decomposition of the active drugs. The most common preservative in ocular solutions are benzalkonium chloride (BAK), chlorobutanol, sodium perborate, thiomersal, disodium edetate, and oxychloro complex (SOC)(6,7). Preservatives can cause toxic epithelial effects and hypersensitivity reactions that range from mild irritation to severe corneal and conjunctival scarring(7). Benzalkonium is known to cause corneal epithelium damage and induce cell membrane lysis at the ocular surface even at very low doses, and, if chosen for treatment, its frequency of use has to be limited to no more than four times a day(8,9).
Preservative-free eye drops prevent these effects and are indicated for severe dry eyes, in patients using multiple preserved drugs, and when higher doses of lubricants are necessary(10,11).
A few clinical trials were performed to compare both types of eye drops in dry eyes. Therefore, this systematic review and meta-analyses focused on whether preservative-free lubricants are more effective than preserved solutions in patients with dry eye disease symptoms.
METHODS
The study has not been submitted to the Research Ethics Committee because it analyzes secondary data that are available in medical literature databases and other similar sources.
This systematic review adhered to the items proposed in The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement(12), without authors’ influence, and was registered at PROSPERO (registration number CRD42018089933).
Literature search
Online search was performed in MEDLINE (via PUBMED), EMBASE, CENTRAL (The Cochrane Controlled Clinical Trials Database), and LILACS databases, from inception to April 2018. We sought for randomized clinical trials that evaluated non-preserved artificial tears versus preserved eye drops as control for the treatment of dry eye disease. The language of literatures was not restricted. Search strategy comprised of the following the terms: lubricants OR artificial tears or artificial tears, lubricants AND dry eye OR dry eye syndrome OR syndromes, dry eye OR dry eyes. We also searched the references of all relevant articles to not miss any study not found in databases.
Inclusion and exclusion criteria
All randomized clinical trials on patients with dry eyes that compared any kinds of preservative-free artificial tears versus any preserved lubricants were included.
Studies performed in healthy eyes or no dry eye; animal studies, or in populations younger than 18 years, or studies with intervention or control was added to other preparations such as corticosteroids and antibiotics were excluded.
The minimum follow-up period for inclusion was 15 days.
Data extraction
Two authors independently screened the titles, abstracts, and full texts to assess whether each article met the eligibility criteria. In the event of disagreement, consensus was used to reach a conclusion. A standardized form was used to compile all study data. Duplicate articles, articles with incomplete data, and those not obtained in full were excluded. If data or a study was not available, the review authors sent a mail to the trial’s authors.
Risk of bias assessment
Two authors independently evaluated the methodological quality of each study assessing the risk of bias in RCTs using the Cochrane’s collaboration tool. Any disagreement was resolved by meeting and discussion to establish a consensus. Selection (random sequence generation and allocation), performance (blinding of participants and personnel), detection (blinding of outcome assessment), attrition (incomplete data), reporting (selective outcomes reporting), and other biases were evaluated.
Outcome analysis
Subjective improvement of dry eye symptoms were the dichotomous outcomes (dryness, scratchiness, foreign body sensation, burning); continuous outcomes were tear film breakup time (TBUT) in seconds, amount of tear film (Schirmer test) in millimeters, corneal staining, corneal or conjunctival epithelium (impression cytology), tear osmolarity, visual acuity, change in the frequency of using the eye drops, and inflammatory biomarker alterations.
Statistical analysis
For dichotomous outcomes, risk ratio and 95% confidence interval (CI) were calculated using a random-effect model (REM). When the effect was not indicated, the risk difference (RD) and 95% CI were calculated using REM. For continuous outcomes, mean and standard deviation were used to generate the mean difference (MD) and 95% CI using REM. The Rev Man 5 statistical package (Cochrane Collaboration) was used to perform meta-analyses. I2 statistical heterogeneity was assessed using heterogeneity tests, i.e., the standard chi-square test (P-value of <0.10 or <10%) and the Higgins test (I2 >50% was statistically significant).
Data synthesis
Assessment of heterogeneity
The I2 statistic was used to assess statistical heterogeneity among studies. An I2 value of >50% was interpreted as indicating substantial statistical heterogeneity.
Reporting bias assessment
A funnel plot was used to assess publication bias and other reporting biases when 10 or more studies were included in the meta-analysis. Asymmetry was interpreted in the funnel plot in conjunction with study characteristics, such as sample size, or other potential factors, such as funding sources.
Sensitivity analysis
When adequate data were available, sensitivity analysis was performed to assess the impact of excluding studies with poor methodological quality, such as lack of allocation concealment, lack of masking, and a large proportion of participants lost to follow-up (≥20%), industry funding, and unpublished studies.
RESULTS
A total of 476 studies were identified in EMBASE, 785 in MEDLINE, 146 in LILACS, and 621 in CENTRAL database. Of the 2028 titles and abstracts, 29 articles were selected. In the last analysis, four clinical trials were retrieved in full text for this review (Figure 1).
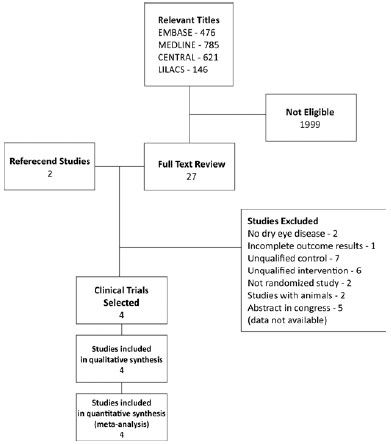
Characteristics of included studies
The characteristics of included studies are summarized in table 1. A total of 323 patients were included in the four selected papers.
One study was conducted in Iran(2), the second was a multicenter study in Colombia, Mexicom and Chile(13), the third study was conducted in Minnesota and New York(14), and the last study took place in Russia(15). The etiology of dry eye disease in most studies was not specific and widely varied (aqueous deficiency or evaporative causes, Sjogren, and no Sjogren syndrome). One study compared non-preserved Tear Naturale (Alcon) with TearLose with BAK as preservative (Darou, Company)(2). The second study compared preservative-free Xiel Ofteno (Sophia Laboratoire) with polidronium chloride preserved Systane (Alcon)(13). The third paper compared unpreserved sodium hyaluronic (Pharmacia, Piscataway-NJ) with Liquifilm (Allergan) with chlorabutanol as conservant(14); and the last article compared preservative-free Hylabak (Abak, Thea Laboratoires) with Systane (Alcon) with polidronium chloride as preservative(15). The sample size ranged from 15 to 76 randomized in each group, and the follow-up period ranged from 1 month to 3 months. The eye drops were used twice a day in a study, four times a day in two studies, and eight times a day in the last study.
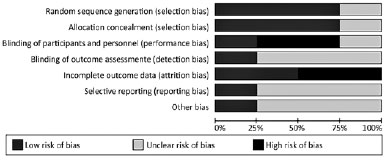
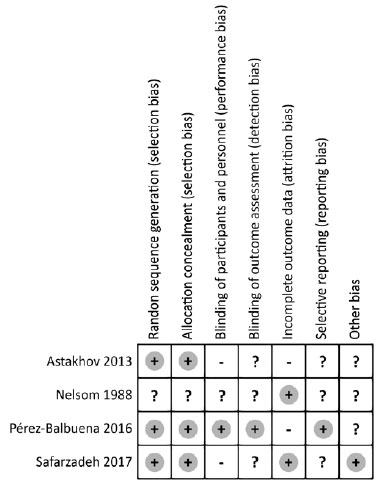
Risk of bias in included studies
The risk of bias assessment of the four included studies are summarized in figures 2 and 3. One study used a random numbers software to prevent selection bias(13), the second used random blocks(2), the third study used a code list allocated to patients in a predetermined order(15), and the last study did not report the random sequence generation(14). The first study was double-blinded using the same label or package bottles(13); the second publication blinded patients using plastic bottles with nozzle droppers and pilfer proof caps(2); the third study blinded the investigators(15); and the last study indicated that it was double-blinded but only reported that medication were packaged in unit-dose plastic containers(14).
Outcome measures
Meta-analysis was possible for three continuous and three dichotomous outcomes. The number of studies used in these results ranged from 2 to 3. Heterogeneity was present in all outcomes, but was only statistically significant for the burning symptom (p=0.003).
Symptoms were evaluated using the Ocular Surface Disease Index- Allergan (OSDI) score in two studies(2,13), without significant differences (MD=0.37, CI 95%: -2.35 to 1.61; p=0.72; I2=0%) (Figure 4). The tear film breakup time (TFBUT) was described in three studies(2,13,14), with MD of 0.20 (CI 95%: -0.85 to 0.44; p=0.54; I2=0%), also without statistically significant difference between the preservative-free and preserved lubricants groups (Figure 5). Aqueous secretion, evaluated using the Schirmer test, was present in three studies, but without statistically significant difference between the intervention and control groups, with the MD of 0.12 (CI 95%: -1.24 to 1.49; p=0.86; I2=0%) (Figure 6)(2,13,14). Corneal staining was evaluated in three studies(2,14,15); however, meta-analysis was not performed because the authors used different methods to quantify this outcome. Burning, foreign body sensation, and photophobia were described in two studies(13,15), for the presence or absence of this symptom, which showed no statistically significant difference between the non-preserved and preserved artificial tears, with RD of 0.07 (CI 95%: -0.17 to 0.30; p=0.59; I2=89%), 0.00 (IC 95%: -0.06 to 0.07; p=0.59; I2=0%) and 0.01 (CI 95%: -0.06 to 0.07; p=0.62; I2=0%), respectively (Figures 7, 8, and 9).
Meta-analysis for other outcomes was not possible because each of them was reported in only one study. Tearing sensation is a symptom described in one study(13), in which the authors described a reduction from 50% to 23% in the intervention group as compared to 28% to 17% in the control group. Hyperemia, a dry eye signal, was also described in the same study(13) and a reduction from 59% to 28% was reported in the intervention group as compared to 73% to 35% in the control group.
Tear osmolarity was evaluated in one study only(14), with the mean of 339.5 mOsm/kg before the use of preservative-free eye drops that changed to 312 mOsm/kg after 8 weeks of intervention in the intervention group, whereas the control group had 371.4 mOsm/kg before and 311.8 after using preserved eye drops. These results were not statistically significant.
Bulbar impression cytology grades were evaluated in one study(14), with the mean of 2.4 before and 2.3 after the intervention as compared to 2.7 before and 2.3 after using the control medication. These results were also not statistically significant.
Palpebral impression cytology grades were evaluated in one study(14), with the mean of 1.3 before and 0.8 after the intervention as compared to 1.0 before and 0.7 after using the control medication, and these results did not show a statistically significant difference.
A visual analog scale to classify pain and discomfort was used in one study(14). The mean score changed from 65.9 to 37 in the intervention group and from 67.6 to 31.3 in the control group, and these results were statistically significant.
Visual acuity was evaluated in two studies; however, the results were not described in one of them(14). In another study, visual acuity was 6/6 in the worst eye in 59.26% of patients at baseline that changed to 81.48% in the intervention group, as compared to 64% to 84% in the control group. These results did not show a statistically significant difference(15).
Flap edema was assessed in one study on after-LASIK dry eye(15), which ranged from 7.41% to none in the intervention group and from 4% to none in the control group. Flap folds were evaluated in the same study, ranging from no case to 3.7% in the intervention group as compared to that in the control group, which showed no flap folds at baseline and after the medication. This study did not report the p-values.
Dryness was evaluated in one study(15), ranging from 3.7% to none in the intervention group as compared to that in the control group, which showed no flap folds at baseline and after the medication. This study also did not report its p-values.
Gritty-eye was evaluated in one study(15), ranging from 11.11% to none in the intervention group as compared to that in the control group ranging from 12% at baseline to zero after the medication use. No p-value description was reported in this study.
Another study evaluated patients with no parallel fold(15), with range from 55.56% at baseline to 81.48% in the intervention group, as compared to 64% to 88% in the control group. This study also did not report its p-values.
One parallel fold was evaluated in one study(15), which ranged from 40.74% at baseline to no case in the intervention group, as compared to 24% to no case in the control group, with no p-value description.
One study assessed the overall efficacy with one of the following answers from the investigator: “satisfactory,” “not very satisfactory,” and “unsatisfactory.” All participants had a “satisfactory” answer from the investigator, except for one participant after the intervention and two patients in the control group at baseline gave a “not very satisfactory” answer regarding the efficacy. After using the medication, only one patient provided the same answer(15).
DISCUSSION
The perfect artificial tear should be able to repair the damaged tear film with less frequency of instillation and with minimal side effects(16-18). The short time duration in the cornea, in addition to the limited time of symptom improvement, is a common problem of lubricants.
Cytotoxicity and high prevalence of ocular surface disorders in preservatives were reported in several studies(19,20). High concentrations of BAK can cause corneal epithelial damage, reduce tear and mucin production, reduce goblet cell density, and induce squamous metaplasia. Recent studies reported that BAK may cause DNA single- and double-strand breaks in human corneal epithelial cells and can affect the blood-aqueous barrier(21-25).
Although preservative-free artificial tears are now recommended for dry eyes, single unit-dose tear substitutes are more expensive for the manufacturers and consumers, and less convenient to use than bottled artificial tear drops(26-28).
This systematic review had some limitations. It compared preserved artificial tears with preservative-free artificial tears, and although all preservatives cause similar ocular surface damages, each one may have particularities.
Another limitation was the various etiologies and severity of dry eye from the study participants, ranging from mild dry eye to severe cases, with Sjogren, no Sjogren, and after Lasik dry eye, which could also influence disease improvement or not with the two groups of lubricants.
The number of participants in each group in selected clinical trials was also a possible limitation, because we included a study with 15 participants in a group(14), as compared to other studies with 75 patients in a group(13).
This systematic review evaluated studies that, if were analyzed individually, no study had low risk of bias; only one of them had more items with low risk of bias.(13) This is an important limitation in this review.
Symptoms and signs are often not compatible with the degree of dry eye disease severity, which makes it difficult to quantify the real improvement of each treatment.
The variables “symptoms” were evaluated in all studies; however, only two of them used the OSDI score(2,13,29). It consists a scale of symptoms, and the greater the score, the more severe is the dry eye disease. No statistically significant difference was observed between the two groups in the OSDI score meta-analysis.
Symptoms were also evaluated using other scales, such as the pain/discomfort analog scale(14), but no statistically significant difference was observed between the two groups. Symptoms were also analyzed individually in two groups; dryness, gritty, foreign body sensation, and photophobia were reduced after the treatment in both groups in one study; however, this study did not mention the statistical significance of the intergroup analysis(15).
Hyperemia and tearing sensation were described in one study(13), and a statistically significant difference was observed between the baseline and after the intervention in each group; however, this study did not describe the p-values between the intervention and control groups. Dryness, gritty, and presence of parallel folds or flap edema (in cases after LASIK surgery) were reported in one study(15), but without p-value description, which was also considered as a limitation in this review (i.e., incomplete outcome data).
Burning, foreign body sensation, and photophobia were described in two studies(13,15), for the presence or absence of this symptom, without statistically significant difference between the preservative-free and preserved artificial tears. Thus, the symptoms were not standardized on a single scale, and they were also not the same in each study when mentioned individually.
The quality of the tear film and its evaporation rate, conducted using the TBUT, and the amount of tear secretion evaluated using the Schirmer test, were analyzed in three studies. Meta-analysis was performed but did not show a statistically significant result.
Corneal and conjunctival staining were assessed in three studies, but one of them only reportedly used a scale grade ranging from 0 to 3(2); another study reportedly graded the lisamine and fluorescein stain but did not describe the methods used, neither the results(13); the third paper used the method by von Bijsterveld grading stain from 0 to 9(14,30); and the fourth one used the Oxford scheme(15,31). The various methods used to perform the staining were a limitation in the meta-analysis of this outcome.
Visual acuity is an important variable, and visual impairment is a common complication in severe cases of dry eyes(28). However, this outcome was reported in two studies only(14,15), in which only one of them had mentioned the results with no statistically significance between control and intervention groups after the treatment.
Tear film osmolarity is known to have an excellent performance in diagnosing dry eyes, which showed higher accuracy than other tests, mainly in severe cases of dry eye disease. However, it was only evaluated in one study, which did not show a statistically significant difference between the two groups(14,32).
Impression cytology is a method performed using a cellulose acetate filter to remove superficial epithelial cells from the conjunctiva to evaluate the degree of metaplasia in the conjunctiva, caused by various etiologies, such as dry eye disease(33). It has very good results in dry eye diagnosis, but was only evaluated in one study, without statistically significant between-group differences(14).
Only one study assessed the “global efficacy”, that authors referred to “patient tolerance” and that this variable was assessed by the investigators, using the terms “not very satisfactory”, “very satisfactory”, and “unsatisfactory”. They reported that only one patient had no tolerance to the control drug in day 28; however, during the last visit (day 84), they did not find a statistically significant results between the groups. The limitation in this outcome is the way the investigators used to classify the patient as satisfactory or not, which was not described in the paper.
With regard to side effects, the reason for the current selection of preservative-free eye drops, no significant adverse effects were observed in any of the groups in these studies. Safarzadeh et al. did not observe any adverse effects in the group that used preserved eye drops and attributed the results to the short treatment time in this study. Perez-Balbuena et al. reported that side effects were not associated with the intervention in their study. Astakhov et al. described that both treatments were well tolerated in their results, without significant topical or systemic adverse effects, or discontinuation of treatment for any reason. Only two cases of corneal edema were reported in the group that used preservative-free lubricants, and one case of corneal edema in the preserved artificial tears group, which were attributed to LASIK surgery and not to the lubricants studied. Finally, Nelson et al. reported that no side effects were observed in any of the groups included in their studies(2,14,15).
In this review, we subjective tests were observed as symptom evaluation, and some of the main objective tests, such as TFBUT and Schirmer, had no significant changes between the two groups. In addition, other tests to diagnose dry eyes, such as tear osmolarity and impression cytology tests, were rarely used in the analyzed studies; this made it difficult to define the improvement between the intervention and control groups.
Therefore, the lack of outcomes, the small number of participants, the control group with various types of conservants, the absence of some primary variables in any studies, the non-standardized method of extracting some outcomes, the various etiologies of dry eye disease in the study participants, and the low quality of studies were a limitation in this systematic review.
Hence, greater effectiveness of one group over the other cannot be proven; therefore, we cannot say that preservative-free artificial tears are better than preserved lubricants for the treatment of dry eye disease. However, we highlight that physicians’ experience is also fundamental in selecting the ideal artificial tears in each patient.
Our results showed that further randomized clinical trials of good quality are required to answer this research question, with the suggestions of improving the related limitations in this review, to achieve better results.
The evidence assessed in the selected clinical trials was not sufficient to prove that preservative-free artificial tears are more effective than preserved artificial tears for the treatment of dry eye disease.
REFERENCES
1. Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017; 15(3):438-510.
2. Safarzadeh M, Azizzadeh P, Akbarshahi P. Comparison of the clinical efficacy of preserved and preservative-free hydroxypropyl methylcellulose-dextran-containing eyedrops. Journal of optometry. 2017;10(4):258-64.
3. Ang BC, Sng JJ, Wang PX, Htoon HM, Tong LH. Sodium hyaluronate in the treatment of dry eye syndrome: A systematic review and meta-analysis. Sci Rep. 2017;7(1):9013.
4. Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002;86(2):181-4.
5. Sullivan BD, Crews LA, Sönmez B, de la Paz MF, Comert E, Charoenrook V, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000-8.
6. Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther. 2001;18(5):205-15.
7. Smith GT, Lee S, Taylor HR. Open evaluation of a new non-preserved artificial tear. Aust N Z J Ophthalmol. 1993;21(2):105-9.
8. Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review. Clin Ophthalmol. 2014;8:1419-33.
9. Jee D, Park SH, Kim MS, Kim EC. Antioxidant and inflammatory cytokine in tears of patients with dry eye syndrome treated with preservative-free versus preserved eye drops. Invest Ophthalmol Vis Sci. 2014;55(8):5081-9.
10. Murube J, Paterson A, Murube E. Classification of artificial tears. In: Sullivan D, Dartt D, Meneray M, editors. Lacrimal gland, tear film, and dry eye syndromes 2. Boston: Springer; 1998. p. 693-704.
11. Lanz R. Comparison of Genaqua®-Preserved Genteal® in Multidose Bottles vs Preservative-Free Tears Naturale® in single dose units in patients with moderate to severe dry eye. Invest Ophthalmol Vis Sci. 2006;47(13):259.
12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
13. Pérez-Balbuena AL, Ochoa-Tabares JC, Belalcazar-Rey S, Urzúa- Salinas C, Saucedo-Rodríguez LR, Velasco-Ramos R, et al. Efficacy of a fixed combination of 0.09% xanthan gum/0.1% chondroitin sulfate preservative free vs polyethylene glycol/propylene glycol in subjects with dry eye disease: a multicenter randomized controlled trial. BMC Ophthalmol. 2016;16(1):164.
14. Nelson JD, Farris RL. Sodium hyaluronate and polyvinyl alcohol artificial tear preparations: a comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol. 1988;106(4):484-7.
15. Astakhov YS, Astakhov SY, Lisochkina AB. Assessment of dry eye signs and symptoms and ocular tolerance of a preservative-free lacrimal substitute (Hylabak®) versus a preserved lacrimal substitute (Systane®) used for 3 months in patients after LASIK. Clin Ophthalmol. 2013;7:2289-97.
16. Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review. Clin Ophthalmol. 2014;8:1419-33.
17. Tong L, Petznick A, Lee S, Tan J. Choice of artificial tear formulation for patients with dry eye: where do we start? Cornea. 2012;31 Suppl 1:S32-6.
18. Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146(3):350-6.
19. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312-34.
20. Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341-9.
21. Chen W, Li Z, Hu J, Zhang Z, Chen L, Chen Y, et al. Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One. 2011;6(10):e26103.
22. Kim JR, Oh TH, Kim HS. Effects of benzalkonium chloride on the ocular surface of the rabbit. Jpn J Ophthalmol. 2011;55(3):283-93.
23. Ye J, Wu H, Zhang H, Wu Y, Yang J, Jin X, et al. Role of benzalkonium chloride in DNA strand breaks in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1681-7.
24. Miyake K, Ota I, Ibaraki N, Akura J, Ichihashi S, Shibuya Y, et al. Enhanced disruption of the blood-aqueous barrier and the incidence of angiographic cystoid macular edema by topical timolol and its preservative in early postoperative pseudophakia. Arch Ophthalmol. 2001;119(3):387-94.
25. Abe RY, Zacchia RS, Santana PR, Costa VP. Effects of benzalkonium chloride on the blood-aqueous and blood-retinal barriers of pseudophakic eyes. J Ocul Pharmacol Ther. 2014;30(5):413-8.
26. Murube J, Murube A, Zhuo C. Classification of artificial tears. II: additives and commercial formulas. Adv Exp Med Biol. 1998; 438:705-15.
27. Climent AO. Comparison of GenAqua-preserved GenTeal in mul-tidose bottles vs preservative-free tears naturale in single dose units in patients with moderate to severe dry eye. Invest Ophthalmol Vis Sci. 2006:47. E-Abstract 261-B439.
28. No authors listed]. Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):163-78.
29. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615-21.
30. Petroutsos G, Paschides CA, Karakostas KX, Psilas K. Diagnostic tests for dry eye disease in normals and dry eye patients with and without Sjögren’s syndrome. Ophthalmic Res. 1992;24(6):326-31.
31. AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640-50.
32. Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35(7):553-64.
33. Nelson JD. Impression cytology. Cornea. 1988;7(1):71-81.
Submitted for publication:
October 2, 2018.
Accepted for publication:
May 14, 2019.
Funding: This study received no specific financial support
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose