

Mert Simsek1; Pinar Cakar Ozdal2; Ali Mert Kocer2
DOI: 10.5935/0004-2749.20190058
ABSTRACT
Purpose: To evaluate the prevalence, clinical characteristics, and types of optic nerve involvement in patients with ocular toxoplasmosis.
Methods: For this retrospective cross-sectional study, we examined all patients with active ocular toxoplasmosis referred to our Uveitis Section during the last 12 years, and we included patients with optic nerve involvement in the study. The primary outcome was the prevalence of optic nerve involvement, and secondary outcomes included the types of optic nerve involvement and the final best-corrected visual acuity after treatment.
Results: The prevalence of optic nerve involvement was 14.4%, with the leading cause being the activation of a juxtapapillary lesion (70.5%). We found papillitis in two eyes and neuroretinitis in two eyes (11.7% for each). We only detected one optic nerve involvement secondary to a distant active lesion (5.8%). Sixteen patients (94.1%) had unilateral ocular toxoplasmosis. The overall final best-corrected visual acuity after treatment was 10/10 (LogMAR = 0.0) excluding the three patients with a juxtapapillary scar involving the macula.
Conclusions: Optic nerve involvement was common in patients with ocular toxoplasmosis. The main type of optic nerve involvement was caused by activation of an old juxtapapillary lesion. Treatment was quickly effective, but the best-corrected visual acuity was dependent on the presence of a scar in the papillomacular bundle.
Keywords: Ocular Toxoplasmosis; Neuroretinitis; Papilledema; Prevalence; Prognosis
RESUMO
Objetivos: Avaliar a prevalência, características clínicas e tipos de acometimento do nervo óptico em pacientes com toxoplasmose ocular.
Métodos: Para este estudo retrospectivo transversal, examinamos todos os pacientes com toxoplasmose ocular ativa encaminhados ao nosso Setor de Uveíte nos últimos 12 anos, e incluímos pacientes com comprometimento do nervo óptico no estudo. O resultado primário foi a prevalência do envolvimento do nervo óptico, e os resultados secundários incluíram os tipos de envolvimento do nervo óptico e a acuidade visual final melhor corrigida após o tratamento.
Resultados: A prevalência de acometimento do nervo óptico foi 14,4%, sendo a principal causa a ativação de uma lesão justapapilar (70,5%). Encontramos papilite em dois olhos e neuroretinite em dois olhos (11,7% para cada um). Apenas detectamos um comprometimento do nervo óptico secundário a uma lesão ativa distante (5,8%). Dezesseis pacientes (94,1%) apresentavam toxoplasmose ocular unilateral. A acuidade visual final com melhor correção após o tratamento foi 10/10 (LogMAR= 0,0) excluindo os três pacientes com uma cicatriz justapapilar envolvendo a mácula.
Conclusões: O comprometimento do nervo óptico foi comum em pacientes com toxoplasmose ocular. O principal tipo de comprometimento do nervo óptico foi causado pela ativação de uma lesão justapapilar antiga. O tratamento foi rapidamente eficaz, mas a acuidade visual final com melhor correção foi dependente da presença de uma cicatriz no feixe papilomacular.
Descritores: Toxoplasmose ocular; Neuroretinite; Papiledema; Prevalência; Prognóstico
INTRODUCTION
Ocular toxoplasmosis (OT) is a parasitic infection caused by Toxoplasma gondii (T. gondii) that usually invades the posterior segment of the eye and is the major cause of infectious posterior uveitis(1) causing 25%-85% of the cases(2).
Ocular involvement in toxoplasmosis is the result of a postnatally acquired infection or a congenital disorder in patients exposed in utero. Diagnosis is based on clinical examination supported by serological tests showing parasitic exposure(3). The patients’ age, immunity status, and the genotype of the parasite also play an important role in the patient’s clinical picture and ocular prognosis(4).
OT signs are most commonly detected in the posterior ocular segment with a retinitis lesion that appears as a white-gray, elevated, and edematous area of retinal tissue frequently adjacent to an old pigmented scar(5). This typical retinal lesion may be associated with varying degrees of vitritis (especially overlying the active retinal lesion) and retinal vasculitis (involving both the retinal veins and arteries) in the posterior segment. Moreover, anterior uveitis associated with large granulomatous keratic precipitates, posterior synechiae, and acute intraocular pressure increases occurs frequently. Also, the lesions may involve the optic nerve directly or develop in its proximity(6,7).
Optic nerve involvement used to be rare(8,9), but large retrospective series have shown higher prevalence rates (5.3% by Eckert et al(10) and 12.9% by Bosch-Driessen et al(11)).
The aims of our study were to describe the demographic characteristics and determine the prevalence and the types of optic nerve involvement in patients with OT and to evaluate the final best-corrected visual acuity (BCVA) after treatment.
METHODS
This retrospective study was conducted with data obtained from January 2006 to December 2017 at the Uveitis Section of Ulucanlar Eye Training and Research Hospital in Ankara, Turkey. The local Ethics Committee approved the study protocol, and we conducted it in accordance with the Declaration of Helsinki. We obtained written informed consents from all participants. All patients were Caucasian-Turkish.
We retrospectively reviewed the medical records of 118 patients diagnosed as having OT. We analyzed data from 17 patients with optic nerve involvement due to active OT at admission or during the follow-up period. We excluded data from patients who were immunocompromized or from those with a previous optic nerve lesion due to causes other than toxoplasmosis, as well as data from records missing information or those with an unclear diagnosis.
We included data from patients with alterations of the optic nerve in the form of a swollen disc or papillitis concomitantly with the presence of an active ocular lesion caused by T. gondii.
The clinical OT diagnoses were based on the criteria defined by Holland et al.(12). Thus, patients with active, cream-white focal retinal lesions accompanied by a hyperpigmented retinochoroidal scar were diagnosed as recurrent OT, and cases without an accompanying hyperpigmented retinochoroidal scar were diagnosed as primary OT. Diagnoses were also confirmed by serological tests specific to T.gondii when necessary.
Diagnoses were based on clinical findings, and we performed serological evaluations (enzyme-linked immunosorbent assay) only on atypical cases (not in patients with typical clinical findings). Positivity for anti-T. gondii IgG antibodies supported the OT diagnoses.
Moreover, we ordered additional examinations such as lung X-rays; tuberculin skin tests; measurement of serum angiotensin-converting enzyme levels; and serological screenings for syphilis, brucellosis, tuberculosis, and human immunodeficiency virus infections to exclude other potential pathologies in cases without typical old pigmented retinochoroidal scars. Complete blood counts, erythrocyte sedimentation rate measurements, and renal and hepatic function tests were performed to determine the general status of the patients.
We classified optic nerve involvement due to OT as published by Eckert et al.(10). Accordingly, we formed four groups: (1) involvement secondary to distant active retinitis lesions (Figure 1), (2) involvement secondary to the activation of juxtapapillary lesions (Figure 2), (3) papillitis (Figure 3), and (4) neuroretinitis (papillomacular or serous macular detachment with hard exudates at the macula and optic nerve involvement) (Figure 4).
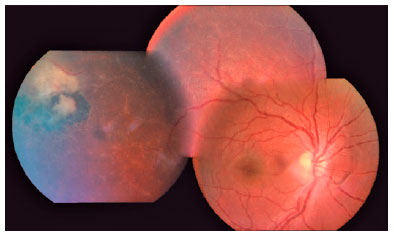
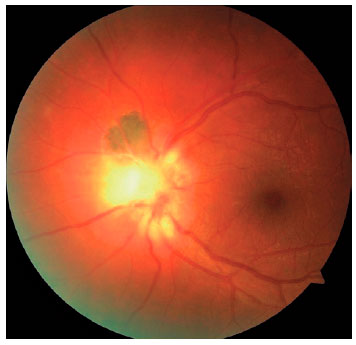
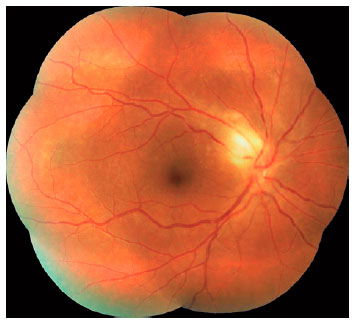
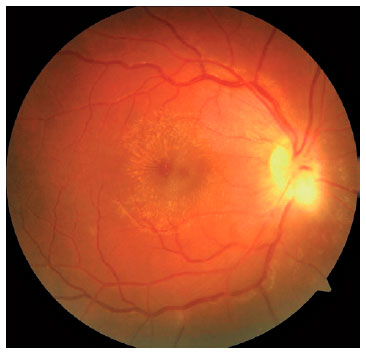
We performed complete ophthalmologic examinations including anterior segment and indirect fundus examinations with a +90 Diopter lens (including color fundus photographies) after dilation in all patients. The examination also included an angiographic evaluation, optic coherence tomography, and Humphrey perimetry as necessary (Figures 5 and 6). We obtained data on age, gender, BCVA at admission, involved eye, laterality, follow-up duration, final BCVA after treatment, and type of optic nerve involvement from the medical records. We measured visual acuity using the ETDRS chart at 6 m. Maximum visual acuity (best-corrected) was determined by correcting the existing refraction, and we measured the final BCVA after treatment and following complete resolution of the active lesion and improvement of optic nerve signs.
We treated all patients following the therapeutic schema applied in our clinic for OT (trimethoprim + sulfamethoxazole 800/160 mg tablet twice daily and azithromycin 500 mg tablet once daily). We added oral corticosteroid therapy (methylprednisolone started at a dose of 0.5 1 mg/kg/day and discontinued by tapering) on the third day of the antiparasitic therapy, and we always discontinued the corticosteroid before stopping the antiparasitic treatment. We examined patients in the active phase at 2 4 week intervals. The treatment durations were based on the clinical statuses and lasted at least 6 weeks.
RESULTS
The optic nerve involvements were detected either at admission or during the follow-up in 17 (14.4%) of the 118 reviewed 118 cases diagnosed as having OT. The mean age of the 17 cases (12 women and 5 men) was 25.35 ± 7.76 years (range, 9 39 years). Their follow-up duration ranged between 2 and 112 months (mean, 33.33 ± 36.10 months). Fifteen cases were diagnosed as having recurrent OT, and two (cases 13 and 14) as having primary OT. We found 12 (70.5%) patients had a lesion (juxtapapillary) in the neighborhood of the optic nerve, 2 (11.7%) had direct involvement (papillitis), 2 (11.7%) had neuroretinitis, and 1 (5.8%) had involvement secondary to a distant active lesion (Table 1). OT was unilateral in the majority of cases (94.1%), and only one case had an old retinochoroidal scar in both ocular fundi (case 1, with a congenital OT infection) with optic nerve involvement in the left eye, but not in the right eye.
The overall mean BCVA value (LogMAR) of the 17 patients with optic nerve involvement was 0.24 ± 0.27 at admission, whereas the mean final BCVA value after treatment was 0.05 ± 0.17. The final BCVA values after treatment were higher than the baseline values in all but four cases (cases 2, 11, 13, and 17), which had no BCVA loss at admission.
Three patients (cases 5, 7, and 9) had an old OT scar located in a juxtapapillary area involving the macula. Although there was an increase in final BCVA after treatment in these cases, the values did not reach the 10/10 level (LogMAR = 0.0). The other patients with juxtapapillary lesions had no scars involving the macula.
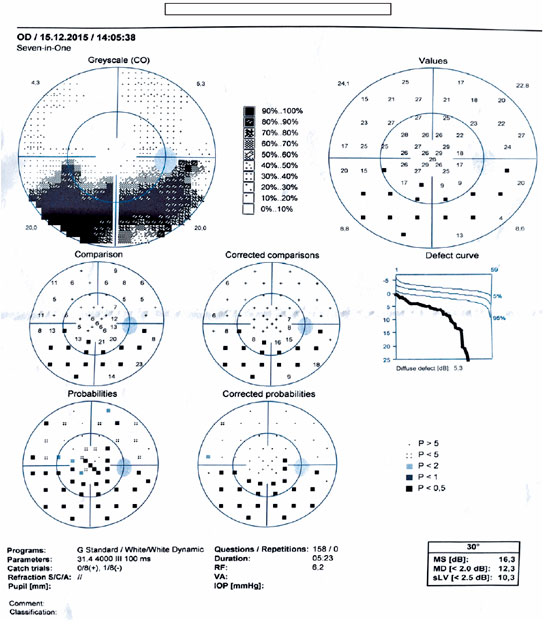
The Humphrey visual field test was performed in the active phase in one patient with papillitis (case 13) and revealed an altitudinal defect in the lower half (Figure 5). Follow-up perimetry revealed that the absolute scotoma in the lower half persisted even after the patient had fully recovered clinically with treatment (Figure 6).
DISCUSSION
The most common OT symptoms are blurred vision and decreased visual acuity, but ocular pain, floaters, and photophobia may also be present more rarely. The most common finding is a white-gray, edematous retinitis lesion with obscure borders in the neighborhood of an old pigmented scar. This phase is frequently accompanied by a dense vitreous inflammation, which may hinder visualization of the lesion (headlight-in-the-fog appearance). In addition, anterior segment involvement characterized by mutton-fat keratic precipitates and posterior synechiae, in addition to vasculitis, retinal hemorrhage, choroiditis, scleritis, and optic nerve involvement, may also accompany the clinical picture. Moreover, OT can rarely lead to neovascularization, pigmentary changes, and retinal detachment(13-18).
Optic nerve involvement is an atypical presentation of OT. It appears as an inflammatory white mass on the optic disc, particularly in the neighborhood of an old pigmented scar or with an accompanying active lesion in the peripheral fundus. Recent epidemiological studies with large case series have focused on optic nerve involvement. A study on 154 patients reported the rate of optic nerve involvement due to OT at 12.9%(11). In a study from Brazil (an endemic region for OT), 926 patients were evaluated and the rate of optic nerve involvement was 5.3%(10). Tutkun et al conducted a study in 109 cases with active OT and reported a frequency of papillitis of 16.5%(19). In our study, the rate of optic nerve involvement was higher at 14.4%.
Toxoplasmosis-associated optic nerve involvement can be divided into three groups: The first one is associated with a distant active lesion, the second one is associated with activation in the neighborhood of a peripapillary old scar, and the third one results from direct optic nerve involvement. In addition, the third group is divided into two subgroups as papillitis and neuroretinitis, depending on the presence/absence of macular involvement. In the largest case series study to date, optic nerve involvement was most commonly encountered as secondary to a distant active lesion, followed by cases secondary to the activation of an old peripapillary scar, and finally by cases resulting from direct involvement(10). In contrast, Tutkun et al reported active lesion sites in the juxtapapillary region or in the region closest to the optic disc in all their patients with optic nerve involvement(19). In our series, the activation of an old pigmented lesion in the juxtapapillary region was the most common type of optic nerve involvement. The time to diagnosis, time to treatment onset, immune status of the patient, and genotypic variety of the parasite may play a role in the different types of optic nerve involvement.
Banta et al determined that the time to diagnosis was delayed but the visual prognosis was better in cases presenting with direct optic nerve involvement without a scar as compared with those presenting with activation of a juxtapapillary scar(20). They attributed the better visual prognosis to the absence of a scar in the peripapillary region and the resultant preservation of the macula. Likewise, Eckert et al determined that the increase in visual acuity was lower in cases with optic nerve involvement secondary to a juxtapapillary lesion as compared with the other groups(10). As the majority of our cases had juxtapapillary lesions, we were unable to make a significant comparison with cases having other types of optic nerve involvement. However, the three cases (cases 5, 7, and 9) in which the post-treatment visual acuity did not increase to the LogMAR = 0.0 level had juxtapapillary lesions. The fundus examination of these patients revealed a pigmented retinochoroidal scar involving the macula. Although OT treatment is effective when provided early, the final BCVA after treatment depends on the presence or absence of a scar in the macula rather than on optic nerve involvement.
Stanford et al suggested that retinochoroidal lesions within one optic disc diameter of the optic disc caused an absolute visual field defect, whereas lesions further away caused a relative scotoma(21). Since the ganglion cells distant to the optic disc represent a wider area in the visual field, absolute scotoma development requires involvement of wider peripheral areas and not of central areas. The post-treatment persistence of these scotomas is thought to be due to remaining full-thickness atrophy despite the resolution of the active lesion. Absolute scotomas persist in the visual field after treatment in cases with optic nerve involvement even when the appearance of the optic disc is back to normal and no atrophy is observed clinically(21). In our patient with papillitis (case 13), the pre-treatment examination of the visual field for the differential diagnosis revealed an altitudinal defect, and the absolute defect persisted after treatment, although the clinical appearance had completely improved.
The prevalence of optic nerve involvement in OT may not be high, and this may lead to a delay in diagnosis of the condition. The optic nerve involvement may also be difficult to distinguish from central retinal vein occlusion and from papillo-phlebitis as their appearance may be similar. Optic nerve involvement in addition to retinal and choroidal involvement needs to be suspected in patients with OT. In our study, the clinical diagnoses were easy because the majority of cases had an old pigmented scar in the neighborhood of the optic disc together with vitreous inflammation. Moreover, we confirmed the clinical diagnoses by serological tests in the cases without accompanying old scars.
We are aware of our study’s limitations including its retrospective design. Also, the group of patients referred to our specialized eye hospital may have included specially difficult and/or treatment-resistant cases, and the higher prevalence of optic nerve involvement may have been biased by this. Thus, our results cannot be generalized to the entire Turkish population.
In conclusion, optic nerve involvement needs to be considered in patients with OT, and the disease should be considered in the presence of localized, protuberant, white lesions of the optic nerve head or margin, regardless of the presence of an old scar.
REFERENCES
1. Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003; 136(6):973-88.
2. Muccioli C, Belfort R. Ocular Toxoplasmosis. In: Pleyer U, Foster CS, editors. Uveitis and immunological disorders. essentials in ophthalmology. Springer, Berlin: Heidelberg; 2007. p. 131-43.
3. Commodaro AG, Belfort RN, Rizzo LV, Muccioli C, Silveira C, Burnier MN Jr, et al Ocular toxoplasmosis: an update and review of the literature. Mem Inst Oswaldo Cruz. 2009;104(2):345-50.
4. Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004; 137(1):1-17.
5. Butler NJ, Furtado JM, Winthrop KL, Smith JR. Ocular toxoplasmosis II: clinical features, pathology and management. Clin Exp Ophthalmol. 2013;41(1):95-108.
6. Folk JC, Lobes LA. Presumed toxoplasmic papillitis. Ophthalmology. 1984;91(1):64-7.
7. Shenoy R, Al Hinai A. Presumed ocular toxoplasmosis presenting as papillitis. Indian J Ophthalmol. 2003;51(4):357-9.
8. Alipanahi R, Sayyahmelli S. Acute papillitis in young female with toxoplasmosis. Middle East Afr J Ophthalmol. 2011;18(3):249-51.
9. Song A, Scott IU, Davis JL, Lam BL. Atypical anterior optic neuropathy caused by toxoplasmosis. Am J Ophthalmol. 2002;133(1):162-4.
10. Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye (Lond). 2007;21(6):746-51.
11. Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. 2002;109(5):869-78.
12. Holland GN, O’Connor GR, Belfort R Jr, Remington JS. Toxoplasmosis. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Ocular infection and immunity. St. Louis (MO): Mosby; 1996. p. 1183-223.
13. Gaynon MW, Boldrey EE, Strahlman ER, Fine SL. Retinal neovascularization and ocular toxoplasmosis. Am J Ophthalmol. 1984;98(5):585-9.
14. Delair E, Latkany P, Noble AG, Rabiah P, McLeod R, Brézin A. Clinical manifestations of ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19(2):91-102.
15. Bosch-Driessen LH, Karimi S, Stilma JS, Rothova A. Retinal detachment in ocular toxoplasmosis. Ophthalmology. 2000;107(1):36 40.
16. Gentile RC, Berinstein DM, Oppenheim R, Walsh JB. Retinal vascular occlusions complicating acute toxoplasmic retinochoroiditis. Can J Ophthalmol. 1997;32(5):354-8.
17. Schuman JS, Weinberg RS, Ferry AP, Guerry RK. Toxoplasmic scleritis. Ophthalmology. 1988;95(10):1399-403.
18. Mets MB, Holfels E, Boyer KM, Swisher CN, Roizen N, Stein L, et al Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122(3):309-24.
19. Tugal-Tutkun I, Corum I, Otük B, Urgancioglu M. Active ocular toxoplasmosis in Turkish patients: a report on 109 cases. Int Ophthalmol. 2005;26(6):221-8.
20. Banta JT, Davis JL, Lam BL. Presumed toxoplasmosic anterior optic neuropathy. Ocul Immunol Inflamm. 2002;10(3):201-11.
21. Stanford MR, Tomlin EA, Comyn O, Holland K, Pavesio C. The visual field in toxoplasmic retinochoroiditis. Br J Ophthalmol. 2005;89(7):812-4.
Submitted for publication:
March 26, 2018.
Accepted for publication:
November 22, 2018.
Approved by the following research ethics committee: Numune Education and Research Hospital, Ankara, Turkey (# E-17-1645)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose