

Felipe Mallmann1,2; Luis Henrique Canani2
DOI: 10.5935/0004-2749.20190055
ABSTRACT
Purpose: To compare the intravitreal concentrations of cellular mediators involved in neurodegeneration, inflammation, and angiogenesis in patients with proliferative diabetic retinopathy and other vitreoretinal diseases.
Methods: A multiplex bead immunoassay was used to measure vitreous levels of pigment epithelium-derived factor, serum amyloid P, C-reactive protein, complement C4, alpha-1 antitrypsin, vascular endothelial growth factor, platelet-derived growth factor-AA, platelet-derived growth factor-BB, interleukin-6, interleukin-8, interleukin-10, tumor necrosis factor alpha and beta in patients undergoing 23-gauge vitrectomy for proliferative diabetic retinopathy and other diagnoses (control group).
Results: We evaluated 55 patients, of whom 24 had proliferative diabetic retinopathy and 31 had other diagnoses including vitreous hemorrhage, retinal detachment, macular hole, and epiretinal membrane. Patients with proliferative diabetic retinopathy demonstrated increased levels of serum amyloid P (85.49 vs. 31.38 ng/mL); C-reactive protein (59.89 vs. 41.75 ng/mL), vascular endothelial growth factor (2,330.11 vs. 554.25 pg/mL; p<0.001), platelet-derived growth factor A (127.32 vs. 39.11 pg/mL), platelet-derived growth factor B (29.37 vs. 7.12 pg/mL), interleukin-6 (69.37 vs. 33.58 pg/mL), interleukin-8 (175.25 vs. 59.71 pg/mL), and interleukin-10 (3.70 vs. 1.88 pg/mL); all p<0.004 when compared with the control group. Levels of pigment epithelium-derived factor (30.06 vs. 27.48 ng/mL; p=0.295), complement C4 (570.78 vs. 366.24 ng/mL; p=0.069), and alpha-1-antitrypsin (359.27 vs. 522.44 ng/mL; p=0.264) were not significantly different between the groups. Intravitreal levels of tumor necrosis factor-alpha and tumor necrosis factor-beta were undetectable. Serum Amyloid P, C-reactive protein, platelet-derived growth factor A, platelet-derived growth factor B, interleukin-6, and interleukin-8 were correlated positively with vascular endothelial growth factor.
Conclusions: Cellular mediators involved in neurodegeneration and inflammation demonstrated increased levels in the vitreous humor of patients with proliferative diabetic retinopathy and may be part of the pathogenesis of diabetic retinopathy.
Keywords: Diabetic retinopathy; Retinal degeneration; Cytokines; Acute phase proteins; Vitreous humor
RESUMO
Objetivo: Comparar as concentrações intravítreas de mediadores celulares envolvidos na neurodegeneração, inflamação e angiogênese em pacientes com retinopatia diabética proliferativa e outras doenças vítreo-retinianas.
Métodos: Um ensaio imunomagnético foi utilizado para medir os níveis vítreos do fator derivado do epitélio pigmentar, amilóide P sérico, proteína-C-reativa, complemento C4, e alfa-1-antitripsina, fator de crescimento do endotélio vascular, fator de crescimento derivado das plaquetas AA, fator de crescimento derivado das plaquetas BB, interleucina-6, interleucina-8, interleucina-10, fator de necrose tumoral alfa e beta em pacientes submetidos à vitrectomia 23-gauge para retinopatia diabética proliferativa ou outros diagnósticos (grupo controle).
Resultados: Foram avaliados 55 pacientes, dos quais 24 tinham retinopatia diabética proliferativa e 31 tinham outros diagnósticos, incluindo hemorragia vítrea, descolamento de retina, buraco macular e membrana epirretiniana. Pacientes com retinopatia diabética proliferativa demonstraram níveis aumentados de amilóide P sérico (85,49 vs 31,38 ng/mL), proteína-C-reativa (59,89 vs 41,75 ng/mL), fator de crescimento do endotélio vascular (2.330,11 vs 554,25 pg/mL, p<0.001), fator de crescimento derivado das plaquetas-A: (127,32 vs 39,11 pg/mL), fator de crescimento derivado das plaquetas-B (29,37 vs 7,12 pg/mL), interleucina-6 (69,37 vs 33,58 pg/mL), interleucina-8 (175,25 vs 59,71 pg/mL) e interleucina-10 (3,70 vs 1,88 pg/mL), todos com p<0,004 quando comparados ao grupo controle. Níveis de fator derivado do epitélio pigmentar (30,06 vs 27,48 ng/mL; p=0,295), complemento C4 (570,78 vs 366,24 ng/mL; p=0,069), alfa-1 antitripsina (359,27 vs 522,44 ng/mL; p=0,264) não foram significativamente diferente entre os grupos. Níveis intravítreos de fator de necrose tumoral alfa e fator de necrose tumoral beta foram indetectáveis. O amilóide P sérico, a proteína C-reativa, o fator de crescimento derivado das plaquetas A e B, a interleucina-6 e a interleucina-8 correlacionaram-se positivamente com o fator de crescimento do endotélio vascular.
Conclusões: Os medidores celulares envolvidos na neurodegeneração e inflamação demonstraram níveis aumentados no humor vítreo de pacientes com retinopatia diabética proliferativa e podem ser parte da patogênese da retinopatia diabética.
Descritores: Retinopatia diabética; Degeneração retiniana; Citocinas; Proteínas da fase aguda; Humor vítreo
INTRODUCTION
Diabetic retinopathy is considered a microvascular complication of diabetes mellitus (DM), with diagnosis and classification based on visible vascular alterations detected on clinical examination. The current clinical treatment aims to stabilize the vascular system, disregarding changes in the neurosensory retina(1,2).
Much evidence from experimental models and humans shows that DM involves a neurodegenerative process related to retinal vascular changes(1,2). Barber et al.(3) demonstrated that DM increases the rate of apoptosis of neural cells, with this rate remaining constant over time, resulting in a cumulative loss with subsequent chronic neurodegeneration. Human studies using optical coherence tomography and electrophysiology have confirmed this hypothesis, exhibiting significant anatomical changes and dysfunctions in the upper retinal layers even before vascular changes can be seen(1,2). Neurodegeneration involves several mechanisms. Changes in neurotrophic factors and neuroinflammation appear to play a key role in the onset and perpetuation of this process(1,2). Growing evidence suggests that inflammatory mediators increase endothelial dysfunction and neurodegeneration in patients with diabetic retinopathy (DR), regardless of vascular endothelial growth factor (VEGF) levels(1,3,4).
In the normal retina, neural tissue is in close contact with the vascular endothelium, forming a neurovascular unit that synthesizes countless trophic factors playing an important role in neuronal and blood-retinal barrier (BRB) homeostasis(1,2). Some of these factors include pigment epithelium-derived factor (PEDF), nerve growth factor (NGF), and brain-derived growth factor(2,5). PEDF is the most distinct of the 3 factors involved in neuroprotection, antipermeability, and antiangiogenesis(2). Other factors in neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, have not been well studied in DR(3). Alpha-1 antitrypsin (A1AT), serum amyloid P (SAP), C-reactive protein (CRP), and complement C4 (CC4) are associated with cell imbalance in these diseases, contributing to a neuroinflammatory process(6-8). Similar to what happens in these diseases, there appears to be a loss of neural homeostasis in DR, thus changing the neurovascular unit(1,2).
Therefore, a better understanding of the neurodegenerative pathogenesis of DR and the development of a therapy focusing on this pathogenesis could hinder its progression to more advanced stages of the disease(1,2). Nevertheless, few studies have evaluated the presence of neurodegenerative/neuroprotective factors in DR in humans(1,2,9). The objective of the present study was to measure the intravitreal levels of different inflammatory and neurodegenerative mediators in patients with proliferative diabetic retinopathy (PDR).
METHODS
We conducted a cross-sectional study of patients undergoing 23-gauge posterior pars plana vitrectomy (PPV) at the Retina Division of the Department of Ophthalmology of the Hospital de Clínicas de Porto Alegre (HCPA) between January 2011 and August 2012.
Inclusion and exclusion criteria
The present study included all patients undergoing 23-gauge PPV at the Retina Division of the HCPA over the study period. Cases consisted of patients with DM and PDR who underwent 23-gauge PPV for vitreous hemorrhage (VH) and/or tractional retinal detachment (RD). Patients without DM undergoing 23-gauge PPV for other reasons were included in the control group. The control group (without DM) comprised patients with VH due to a different etiology (n=12), RD (n=10), and maculopathy (macular hole [MH] and epiretinal membrane [ERM], n=9). We excluded patients meeting the following criteria: (1) the presence of silicone oil (12); (2) active uveitis (8); (3) photocoagulation in the past 3 months (none); (4) the use of intravitreal corticosteroids in the past 6 months (none); (5) the use of intravitreal anti-VEGF therapy in the past 3 months (none); and (6) refusal to participate in the study (none).
Surgeries were performed according to the clinical indications of the patients. Our study was approved by the Research Ethics Committee of the HCPA in accordance with the principles of the 2013 Declaration of Helsinki. All patients signed a written consent form before undergoing surgery.
Collecting and handling the material
All surgical procedures and collection of material were performed by the same researcher (FM). The capillary glucose test was performed in patients with diabetes according to the hospital protocol after admission. A 1.5-mL sample of vitreous humor was collected immediately before the surgical procedure and divided into 3 Eppendorf tubes of 0.5 mL each. The samples were properly cooled at 4°C, labeled, and immediately stored in a freezer at -80°C. In February 2014, the first Eppendorf tube of each sample containing undiluted vitreous material (0.5 mL) was thawed.
Material analysis
After being thawed, the samples were analyzed using a bead-based multiplex immunoassay. The assay was performed by a trained technician according to the manufacturer’s instructions (Multiplex; Milliplex MAP, EMD Millipore Corporation, Billerica, MA, USA) using a Luminex xMAP 100/200 analyzer (Luminex Corporation, Austin, TX, USA).
We used preset kits from Multiplex (Multiplex Milliplex MAP Kit) to measure 13 analytes as follows: (1) neurodegenerative panel (HNDG2MAG-36k): PEDF, CRP, CC4, A1AT, and SAP; and (2) inflammatory panel (HCYTOMAG-60k): platelet-derived growth factor (PDGF)-AA and -BB, VEGF, IL-6, IL-8, IL-10, TNF-alpha, and TNF-beta. Before each analysis, the Luminex xMAP analyzer was calibrated according to the manufacturer’s instructions. Standard curves for each plate were calculated with 7 points, starting with the background (0 ng/mL or pg/mL), followed by the minimum detection value (3.2 ng/mL or pg/mL) up to 10,000 ng/mL or pg/mL (16, 80, 400, and 2,000 ng/mL or pg/mL). We found that r2 was higher than 0.99 in all curves.
All samples were analyzed twice, and the mean value was determined using XPONENT computer software (XPONENT, Luminex Corporation). If one of the measures had an error, the other value was used for statistical purposes. When both values were outside the detection limit of the kit (<3.2 pg/mL or ng/mL or >10,000 pg/mL or ng/mL), the detection threshold was calculated for statistical analysis.
Statistical analysis
We utilized the resources provided in the literature and a difference of ±100 pg/mL in measuring VEGF to calculate the sample size considering 2 groups and using a computer program (WinPepe, version 4.0). To achieve 90% power with 5% alpha error (p<0.05), the number of participants in each group was set at 23 (a total of 46). SPSS for Statistics, version 16 (SPSS Inc., Chicago, IL, USA), was used for appropriate calculations.
The results are expressed as means, standard deviations, medians, quartiles, and minimum and maximum values. The Kolmogorov-Smirnov test was conducted to assess normality of data (Gaussian distribution). All analytes showed a non-normal distribution; therefore, logarithmic transformation was performed, and the Student t-test was used to compare cases and controls. The Kruskal-Wallis test was performed to compare the different diagnostic groups: (1) PDR; (2) VH; (3) RD; and (4) maculopathy. The Pearson correlation coefficient was used for correlation analysis.
RESULTS
A total of 55 patients (mean age, 52.5 ± 17.1 years) were included in this study. Most of the patients were white (91.9%) and male (62.9%). Twenty-four patients underwent vitrectomy due to PDR and 31 because of other diagnoses. No statistical difference in demographic data was seen between the groups (Table 1).
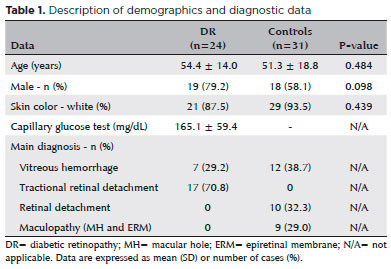
Acute phase proteins, CRP (59.89 ± 23.22 vs. 41.75 ± 28.53 ng/mL; p=0.003), and mainly SAP (85.49 ± 97.30 vs. 31.38 ± 57.92 ng/mL; p=0.001) were increased in patients with PDR when compared with controls. However, levels of PEDF (30.06 ± 8.56 vs. 41.75 ± 28.53 ng/mL; p=0.295), CC4 (570.78 ± 1,153.39 vs. 366.24 ± 990.27 ng/mL; p=0.069), and A1AT (59.89 ± 23.22 vs. 41.75 ± 28.53; p=0.264) were similar between patients with PDR and controls (Figure 1).
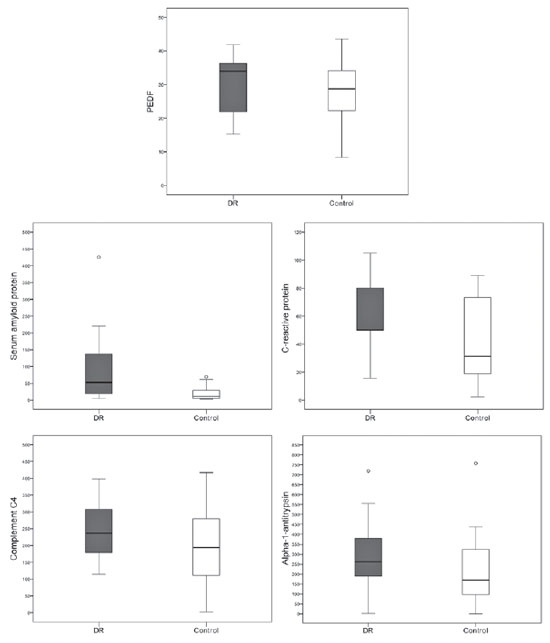
Patients with PDR had intravitreal levels of IL-6 (69.37 vs. 33.58 pg/mL), IL-8 (175.25 vs. 59.71 pg/mL), and IL-10 (3.70 vs. 1.88 pg/mL), which were higher than those in the control group (all p<0.004). Both PDGF isoforms (AA and BB) were also different between the groups, with increased values in patients with PDR (127.32 vs. 39.11 pg/mL and 29.37 vs. 7.12 pg/mL). Patients with PDR had higher VEGF levels than controls (2,330.11 ± 4,037.33 vs. 554.25 ± 4,037.33 pg/mL; p<0.001). In nearly all of the samples, TNF-alpha and -beta levels were below the detection threshold; therefore, statistical analysis was not possible (Figure 2).
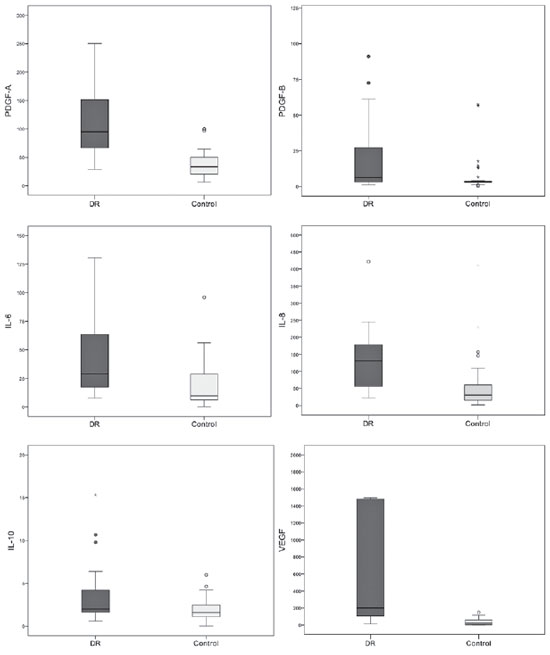
When considering the control group and comparing the different diagnoses with patients with PDR, we identified differences only in PDGF-B, IL-6, and IL-10, which remained at increased levels in the latter, followed by RD, VH due to other etiologies and maculopathy (all p<0.02).
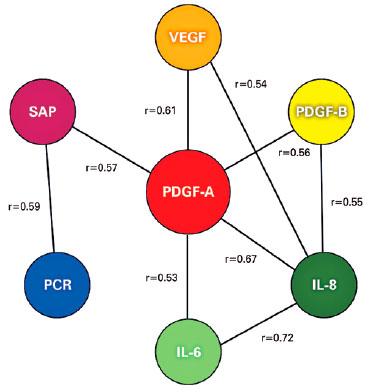
SAP was correlated positively with CRP (r=0.59; p<0.001), A1AT (r=0.33; p=0.013), and PEDF (r=0.30; p<0.001). PEDF was correlated positively with A1AT (r=0.35; p=0.009), and CC4 was not correlated with any of the proteins investigated. A positive correlation existed between IL-6 and IL-8 (r=0.724; p<0.001), IL-6 and IL-10 (r=0.316; p=0.019), and IL-8 and IL-10 (r=0.317; p=0.018). IL-6, IL-8, and IL-10 were also correlated positively with PDGF-A (r=0.532; p<0.001), (r=0.668; p<0.001), and (r=0.317; p=0.018) and PDGF-B (r=0.283; p=0.036), (r=0.553; p<0.001), and (r=0.285; p=0.035), respectively. PDGF-A correlated with IL-6 (r=0.532; p<0.001), IL-8 (r=0.668; p<0.001), and IL-10 (r=0.317; p=0.018); whereas PDGF-B correlated with IL-6 (r=0.283; p=0.036), IL-8 (r=0.553; p<0.001), and IL-10 (r=0.285; p=0.035). VEGF was correlated positively with CRP (r=0.43; p=0.001), SAP (r=0.46; p<0.001), and all cytokines except for IL-10, A1AT, CC4, and PEDF (Figure 3).
DISCUSSION
In the present study, we identified an increase in numerous factors involved in neurodegeneration and inflammation in patients with PDR. Pentraxins (SAP and CRP), PDGF-A and PDGF-B, and IL-6 and IL-8 were consistently increased in patients with PDR and correlated with VEGF, thus emphasizing their neuroinflammatory role in PDR.
The imbalance between angiogenic/inflammatory mediators and neuroprotective factors plays a key role in the onset and perpetuation of damage to the neurovascular unit in DR(2,10). Among the mediators described in the literature, VEGF is essential in the pathogenesis of PDR. VEGF blockade via intravitreal injections of medications altered the treatment of patients with this complication(10,11). As expected, the median VEGF levels in our study were approximately 10-fold higher in patients with PDR than in controls. VEGF is a potent angiogenic agent that promotes vascular permeability(10,11). However, as a survival factor for photoreceptor cells, endogenous VEGF is involved in the maintenance and function of retinal neurons(12). Therefore, it is important to consider the neuroprotective effects of this cytokine, especially in the continued treatment of diabetic macular edema and PDR using anti-VEGF therapies(2).
PDGF is necessary for vessel maturation and pericyte survival(13). We found increased levels of PDGF-A and PDGF-B in patients with PDR that were also correlated positively with VEGF and other interleukins (IL-6, IL-8, and IL-10). Few authors have measured PDGF levels in patients with DR(9). Cassidy et al. reported detectable intravitreous levels of PDGF in patients with VH and RD with proliferative vitreoretinopathy (PVR) and intraocular foreign bodies, but not in RD without PVR(14). When stratifying our control group, we found increased levels of PDGF in VH and RD with PVR compared with levels in maculopathy; however, our PDR group had even higher levels. Praidou et al. found that levels of all PDGF isoforms in the vitreous humor were significantly increased in patients with PDR and did not correlate with serum levels, implying that most of it is part of intraocular synthesis and not serum diffusion(15). Therefore, similar to other vasoproliferative eye diseases, PDGF appears to be part of DR pathogenesis, and its blockade could act synergistically with anti-VEGF therapy, reducing fibrovascular formation and avoiding tractional RD and/or VH(1,16).
The chemoattractant and immunomodulatory properties of IL-6 and IL-8 are important to the immune system. Evidence also suggests that IL-6 and IL-8 stimulate angiogenesis and increase vascular permeability. Our research, as well as other studies, found increased levels of these interleukins in isolated investigations(17,18) and, more recently, in multi-assay analysis(4), thus confirming their role in the pathogenesis of the disease.
IL-10, in turn, has anti-inflammatory properties that suppress immune cells and inhibit the action of other cytokines(19). Few studies have evaluated this cytokine in patients with PDR(4,9). We demonstrated that IL-10 is increased in the vitreous humor of patients with PDR. Moreover, this finding was also reported by other authors(4), and we believe that it is probably part of a compensatory mechanism involving a chronic inflammatory process in an attempt to preserve local homeostasis(20).
Experimental models have illustrated that injection of TNF-alpha generates an intraocular inflammatory process, and its presence is important in the breakdown of the BRB in DR(21). We did not detect intravitreal levels of TNF-alpha and TNF-beta in the present study, and Bromberg et al.(4) reported a similar result. However, Demircan et al. identified increased serum and intravitreal levels of TNF-alpha in patients with PDR when compared to control corpses(22). We do not know if the lack of intravitreal levels in our sample was due to a limitation of the kit, protein lability, or whether TNF-alpha and -beta are not significantly increased in patients with PDR.
PEDF is a neurotrophic factor providing neuroprotective, antiangiogenic, vascular antipermeability, and antioxidant properties(23). PEDF protects neurons against glutamate-mediated excitotoxicity and reduces oxidative stress and inflammation produced by high glucose levels(23). Some authors(9) found reduced intravitreal levels in patients with PDR, while Duh(24) detected increased levels. In the present study, patients with PDR and the control group had similar intravitreal levels. We do not know if this finding is due to the fact that our control group was heterogeneous, consisting of patients with eye diseases, such as RD and VH from other causes, which could change the intravitreal levels of PEDF. Another possibility is a compensatory increase in PEDF in an attempt to counterbalance the effects of VEGF and other inflammatory cytokines. For ethical reasons, all studies involving humans were based on non-normal controls, selecting macular diseases with an indication of vitrectomy, but little angiogenic and inflammatory activity, such as MH and ERM. In our study, we decided to keep groups of angiogenic and inflammatory potential because our objective was to assess the difference between PDR and other vitreoretinal diseases and compare the different diseases to emphasize the specific characteristics of PDR. However, no differences were seen even after stratifying the values according to diagnosis.
CRP and SAP belong to the subfamily of short pentraxins that are involved in the acute-phase inflammatory response associated with innate immunity(25). CRP is involved in endothelial dysfunction and atherogenesis, is associated with non-ocular macrovascular and microvascular complications related to DM(26). SAP is associated with increased cicatricial repair in neurodegenerative diseases, and experimental studies have demonstrated the direct neurotoxicity of SAP with neuronal apoptosis and microglial activation, promoting an increase in pro-inflammatory cytokines(8). Our study is one of the few to report increased intravitreal CRP and SAP levels in patients with DR(27), emphasizing the local neuroinflammatory component in this complication of DM.
CC4 is an essential component of the effector phase of the humoral immune response. Gerl et al.(28) reported evidence of extensive complement activation in the choriocapillaris in the eyes of patients with diabetic. In our sample of patients with PDR, C4 levels were similar to those of the control group, suggesting that there is no difference between PDR and these diseases regarding the role of C4. However, this does not exclude the role of other pathways or complement factors in the pathogenesis of DR.
Although A1AT is involved in neurodegenerative diseases(6), its intravitreal levels were similar between patients with PDR and the groups evaluated. The literature does not mention studies analyzing the serum or intravitreal levels of A1AT in patients with DR without a definition of its role in the disease(9).
Our study has some limitations that should be considered when interpreting the results. We did not evaluate other cytokines or proteins involved in neuroprotection, such as NGF, brain-derived neurotrophic factor, insulin, or erythropoietin. We sought to evaluate the difference between PDR and other diseases; however, in our sample of controls, most diseases had a neurodegenerative potential (ischemic retinopathies and RD), possibly minimizing the differences between groups. In addition, we did not have sample power to detect such differences between the diagnostic groups because the sample size calculation was performed based on 2 groups and using VEGF. In agreement with our sample, the literature demonstrates that most cytokines and mediators have a non-Gaussian distribution, limiting our power to rule out differences when they actually exist (type 2 error); this may be particularly important regarding mediators such as PEDF, CC4, and A1AT. In addition, the absence of differences does not exclude the role of these factors in the pathogenesis of DR; it only suggests a specific mechanism of this DM complication.
Finally, we aimed to evaluate the intravitreal levels of neurodegenerative, inflammatory, and angiogenic mediators in PDR. Our findings revealed that these factors independent of VEGF could be involved in the pathogenesis of DR, suggesting that it should be considered a neurovascular degenerative process rather than solely a microvascular complication of DM. Therefore, in addition to anti-VEGF treatment, alternative and multitargeted therapy should be developed to better control and prevent DR(29).
REFERENCES
1. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227-39.
2. Ola MS, Nawaz MI, Khan HA, Alhomida AS. Neurodegeneration and neuroprotection in diabetic retinopathy. Int J Mol Sci. 2013;14(2):2559-72.
3. Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156-63.
4. Bromberg-White JL, Glazer L, Downer R, Furge K, Boguslawski E, Duesbery NS. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Invest Ophthalmol Vis Sci. 2013;54(10):6472-80.
5. Villarroel M, Ciudin A, Hernández C, Simó R. Neurodegeneration: an early event of diabetic retinopathy. World J Diabetes. 2010;1(2):57-64.
6. Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency - a model for conformational diseases. N Engl J Med. 2002 Jan;346(1):45-53.
7. Orsini F, De Blasio D, Zangari R, Zanier ER, De Simoni MG. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci. 2014;8:380.
8. Kolstoe SE, Ridha BH, Bellotti V, Wang N, Robinson CV, Crutch SJ, et al. Molecular dissection of Alzheimer’s disease neuropathology by depletion of serum amyloid P component. Proc Natl Acad Sci USA. 2009;106(18):7619-23.
9. McAuley AK, Sanfilippo PG, Hewitt AW, Liang H, Lamoureux E, Wang JJ, et al. Vitreous biomarkers in diabetic retinopathy: a systematic review and meta-analysis. J Diabetes Complications. 2014;28(3):419-25.
10. Abcouwer SF. Angiogenic Factors and Cytokines in Diabetic Retinopathy. Journal of clinical & cellular immunology. 2013; Suppl 1(11).
11. DRCRnet. Comparative Effectiveness Study of Intravitreal Aflibercept, Bevacizumabe and Ranibizumabe for Diabetic Macular Edema. Clinical Trials.gov NCT01627249 2013.
12. Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, et al. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS One. 2008;3(11):e3554.
13. Campochiaro PA. Ocular neovascularization. J Mol Med (Berl). 2013;91(3):311-21.
14. Cassidy L, Barry P, Shaw C, Duffy J, Kennedy S. Platelet derived growth factor and fibroblast growth factor basic levels in the vitreous of patients with vitreoretinal disorders. Br J Ophthalmol. 1998;82(2):181-5.
15. Praidou A, Klangas I, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, et al. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009;34(2):152-61.
16. Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006; 168(6):2036-53.
17. Nakamura N, Hasegawa G, Obayashi H, Yamazaki M, Ogata M, Nakano K, et al. Increased concentration of pentosidine, an advanced glycation end product, and interleukin-6 in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2003;61(2):93-101.
18. Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Takeuchi M, Goto H, et al. Correlation of vascular endothelial growth factor with chemokines in the vitreous in diabetic retinopathy. Retina. 2010;30(2):339-44.
19. Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21(5):315-24.
20. Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011;1373:189-94.
21. Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, et al. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52(3):1336-44.
22. Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond). 2006;20(12):1366-9.
23. He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond). 2015; 128(11):805-23.
24. Duh EJ, Yang HS, Haller JA, De Juan E, Humayun MS, Gehlbach P, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol. 2004;137(4):668-74.
25. Vilahur G, Badimon L. Biological actions of pentraxins. Vascul Pharmacol. 2015;73:38-44.
26. Sasongko MB, Wong TY, Jenkins AJ, Nguyen TT, Shaw JE, Wang JJ. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabet Med. 2015; 32(5):686-91.
27. Kimura K, Orita T, Kobayashi Y, Matsuyama S, Fujimoto K, Yamauchi K. Concentration of acute phase factors in vitreous fluid in diabetic macular edema. Jpn J Ophthalmol. 2017;61(6):479-83.
28. Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2002;43(4):1104-8.
29. Simó R, Hernández C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res. 2015;48:160-80.
Submitted for publication:
May 25, 2018.
Accepted for publication:
October 23, 2018.
Approval by the following research ethics committee: Hospital de Clínicas de Porto Alegre (CAAE: 143734.13.8.0000.5327)
Funding: This study was supported by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose