

Gleyse Karina Lopes de Oliveira Pinheiro1; Irami de Araújo Filho2; Irami de Araújo Neto3; Amália Cinthia Meneses Rêgo4; Eduardo Pereira de Azevedo4; Francisco Irochima Pinheiro4; Acácio Alves de Souza Lima Filho5
DOI: 10.5935/0004-2749.20180086
ABSTRACT
Nature has always provided an unlimited source of biologically-active compounds. Since the beginning of mankind, humans have sought resources in fauna and flora to treat eye diseases. However, it was only after the Industrial Revolution that extracts of plants and substances of animal origin could be used safely, as has been determined by controlled interventional studies. Two major challenges faced by ocular pharmacology are the following: developing drugs that are able to reduce blindness due to glaucoma; and controlling the pain associated with eye surgery. The search for a drug that effectively lowers intraocular pressure and controls the progression of glaucoma has led to the development of various ocular hypotensive agents, such as physostigmine from the Physostigma venenosum plant. The anesthetic properties of cocaine, extracted from Erythroxylon coca, finally enabled surgical procedures in the eye. Several new natural compounds have been investigated in an attempt to identify substances with the potential to provide additional benefits to eye tissue and vision. Emerging evidence of anti-inflammatory, wound-healing, antimicrobial, antioxidant, antitumor, and antiangiogenic properties attributed to plant extracts and animal tissues has encouraged more investment in research in this area. Despite technological advances in synthesizing drugs, the pharmaceutical industry still seeks new active compounds from natural sources as well as from revisiting already-established naturally derived compounds. Although a large number of naturally-occurring compounds is known, this review article focuses on the bioactive substances with scientifically-proven benefits for ocular tissues.
Keywords: Nature; Plants, medicinal; Molecules; History of medicine; Pharmacology; Eye diseases; Pharmaceutical industry
RESUMO
A natureza sempre se forneceu uma fonte inesgotável compostos biologicamente ativos. Desde o início da humanidade, os homens buscaram recursos na fauna e flora para tratar as doenças oculares. Porém, foi somente após a Revolução Industrial que extratos de plantas e substâncias de origem animal puderam ser utilizados com segurança, como foi determinado por estudos controlados de intervenção. Dois grandes desafios enfrentados pela farmacologia foram: desenvolver drogas capazes de reduzir a cegueira pelo glaucoma; e controlar a dor associada à cirurgia ocular. A busca por uma droga que efetivamente reduza a pressão intraocular e controle a progressão do glaucoma levou ao desenvolvimento de diversos hipotensores oculares, como a physostigmine da planta Physostigma venenosum. As propriedades anestésicas da cocaína, extraídas da Erythroxylon coca, finalmente permitiram procedimentos cirúrgicos no olho. Vários novos compostos naturais foram investigados na tentativa de identificar substâncias com potencial para fornecer benefícios adicionais ao tecido ocular e à visão. Evidências emergentes de propriedades anti-inflamatórias, de cicatrização de feridas, antimicrobianas, antioxidantes, antitumorais e antiangiogênicas atribuídas a extratos de plantas e tecidos animais estimularam mais investimentos em pesquisas nessa área. Apesar dos avanços tecnológicos na síntese de drogas, a indústria farmacêutica ainda busca novos princípios ativos a partir de fontes naturais, bem como revisita drogas derivadas já estabelecidas. Embora um grande número de compostos que ocorrem naturalmente seja conhecido, este artigo de revisão concentra-se nas substâncias bioativas com benefícios cientificamente comprovados para os tecidos oculares.
Descritores: Natureza; Plantas medicinais; Moléculas; História da medicina; Farmacologia; Doenças oculares; Indústria farmacêutica
INTRODUCTION
The use of herbal extracts to treat ophthalmic conditions dates back to ancient times. The topical use of the macerated fruit of Atropa belladonna by the Egyptians is the first known use of a nature-derived agent to treat an ophthalmic disease(1). However, the emergence of safer and more effective ophthalmic drugs depended on scientific studies in the areas of physiology and pharmacology as well as on technological developments in the pharmaceutical industry during the 19th and 20th centuries(2).
The discovery of drugs capable of reducing intraocular pressure (IOP), thereby controlling the progression of glaucoma, was one of the most important research findings that stimulated interest in molecules extracted from plants(1). The search for a natural drug capable of anesthetizing ocular tissues ran in parallel with the discovery of antiglaucoma drugs(2). The discovery of the anesthetic properties of cocaine, a milestone in the history of ocular pharmacology, only occurred in the late 19th century(2). From then on, exponential growth in the arsenal of plant-derived drugs has been observed, partially due to the growth of the pharmaceutical industry as it continues seeking new discoveries and new markets.
Even 155 years after the introduction of physostigmine as the first herbaceous drug with ocular hypotensive activity, the pharmaceutical industry still considers nature an inexhaustible source of new substances with therapeutic potential(3,4). Despite technological innovation that allows the synthesis of new drugs, biologically-active molecules extracted from plants and animal tissues continue to stimulate extensive research and investment. However, many of these naturally-occurring compounds have yet to be rigorously investigated for activity in ocular tissues. Therefore, in this current review, we have selected natural compounds with a substantial body of evidence in the current scientific literature from controlled studies supporting their use in ophthalmology.
HISTORICAL PERSPECTIVE
Physostigma venenosum and glaucoma
Glaucoma is the leading ophthalmic disease that stimulates the search for new nature-derived drugs. In 1622, Richard Bannister associated vision loss in glaucoma with elevated levels of IOP. In 1885, Adolf Weber postulated the concept of glaucoma as an optic neuropathy. Since then, a number of drug candidates with ocular hypotensive properties have been investigated5.
In 1864, physostigmine, the first herbal remedy against the progression of glaucoma, was identified(3). This alkaloid was isolated from Calabar beans, seeds of the plant P. venenosum(5). Also referred to as eserin, physostigmine is an acetylcholinesterase inhibitor. After instillation in the eye, it induces miosis by increasing the activity of free acetylcholine on the pupil sphincter, leading to accommodation by contraction of the circular portion of the ciliary muscle. The ocular hypotensive effect reported by Laqueur and Weber in 1876 was due to increased drainage of the aqueous humor from the trabecular pathway that resulted from the contraction of the longitudinal portion of the ciliary muscle, as well as from traction on the scleral spur and expansion of the iridocorneal angle (Figure 1)(5). The isolation of physostigmine represents the initial step toward the creation of a class of ocular hypotensive agents also called miotics. However, physostigmine causes several side effects after instillation in the eye, including headache, spasm of accommodation, blurred vision due to miosis, increased risk of retinal detachment, and inflammation of the conjunctiva, cornea, and iris. Therefore, it has been replaced by safer, more effective drugs with fewer side effects(3,4).
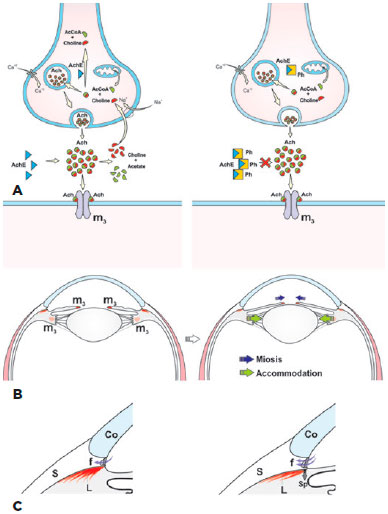
Pilocarpine, the second miotic drug with the ability to reduce IOP, was introduced by Weber in 1876 as therapy for glaucoma. Isolated from the leaves of Pilocarpus jaborandi, a shrub found in subtropical regions of Brazil, pilocarpine acts directly on the muscarinic receptors of the pupil sphincter and on the ciliary muscle (Figure 2)(5). By comparison with other parasympathomimetic drugs, pilocarpine has more tolerable side effects, so its use has continued to the present.
Erythroxylon coca and topical ocular anesthesia
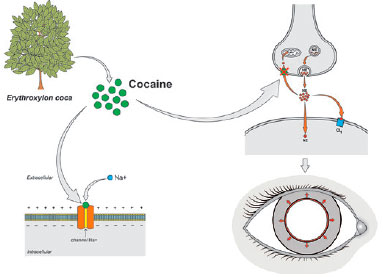
The side effects of E. coca, considered a divine plant by the indigenous people of Peru, aroused the interest of Europeans during the conquest of Peru by Francisco Pizzaro in 1530. The stimulant effects of chewing E. coca leaves were evident among the indigenous people, who referred to it as Kok. In 1850, the Austrian Carl von Scherzer took a considerable amount of the plant to Europe for a more detailed analysis by the chemists Albert Niemann and Wilhelm Lossen. In 1860, Niemann was finally able to isolate the alkaloid from E. coca, which he named cocaine(2).
Almost three decades had passed since the introduction of cocaine for clinical use when Sigmund Freud, in his treatise Über Coca, discussed the anesthetic properties of cocaine(6,7). However, it was Karl Köller, a young Austrian physician colleague of Freud’s at the Royal General Hospital of Vienna who undertook the first use of cocaine for topical ocular anesthesia in 1884. This introduction of ophthalmic anesthesia opened the way for ocular surgical procedures(7,8).
As a local anesthetic, cocaine acts by blocking the sodium channels in the membranes of nerve endings, which prevents nerve transmission. In addition, by inhibiting norepinephrine reuptake at the synapses, cocaine acts as an adrenergic agonist, causing mydriasis and conjunctival vasoconstriction (Figure 3). Cocaine hydrochloride was formulated in 1% and 4% aqueous solutions, yielding complete corneal anesthesia 20 min after instillation. The effect lasted 1 to 2 h. However, cocaine was highly toxic to the corneal epithelium, leading to its abandonment for this purpose(6,7).
Despite cocaine’s rapid rise and abrupt fall, the pioneering studies into its properties prompted the pharmaceutical industry to develop new, safer, and better tolerated local anesthetics for ophthalmic use, such as tetracaine, proparacaine, and lidocaine(2).
OPHTHALMIC PRODUCTS OF HERBAL AND ANIMAL ORIGIN
Research into compounds from a variety of flora and fauna that might serve as drug prototypes for treatment or prevention of various ophthalmic diseases has continued (Table 1).
Aloe vera
A. vera (also called A. barbadensis Miller), a plant native to tropical climates, has juicy leaves and a crown arrangement. Its taxonomic name has become its common name in English, referred to simply as aloe vera. It belongs to the Liliaceae family and has a variety of pharmacologic properties, such as wound-healing, bactericidal, immunomodulatory, antiviral, anti-inflammatory, antioxidant, and antifungal effects(9). Extracts of A. vera contain a number of compounds, including flavonoids, anthraquinones, phenolic acids, enzymes, and vitamins. Investigating these effects of these extracts on human corneal cells, Worzniak and Paduch showed that ethanolic and ethyl acetate may be of benefit in inflammatory ocular diseases, as they have low toxicity and immunomodulatory activity(10). In another in vitro study, Curto et al. observed that aloe vera solution may have the potential to accelerate re-epithelialization and decrease fibrosis in superficial corneal lesions(9). Similarly, Atiba et al. showed that aloe vera eye drops (60 mg/mL) three times a day for three days led to better re-epithelialization and a less intense inflammatory response in a rat cornea model of an alkali burn(11).
Caffeine
Caffeine is an alkaloid consumed worldwide in coffee(12). Coffee powder comes from roasting and grinding beans from two main species of plants: Coffea arabica and C. canephora. Caffeine solution in the form of eye drops is very stable and resistant to photodynamic degradation. It has therefore been tested for the prevention of cataracts by providing ultraviolet (UV) protection. Varma et al. demonstrated both in vitro and in vivo that caffeine reduced the development of cataracts in the rat crystalline lens(13,14). Similarly, Kronschläger et al. showed that administration of caffeine eye drops with 0.9% hydroxypropylmethyl cellulose had a protective effect against cataracts induced by UV-B-type radiation exposure in rats(12). A different property was demonstrated by Zhang et al., who found a protective effect of caffeine against oxygen-induced retinopathy in mice(15). Caffeine added to drinking water ingested by lactating females and the pups themselves selectively attenuated pathologic hypoxia-induced angiogenesis without interfering in the normal development of retinal vascularization. Chen et al. reviewed the experimental evidence for caffeine’s action as a non-selective antagonist of the adenosine receptors A1R, A2AR and A2BR, which are involved in oxygen-induced retinopathy(16).
Cannabinoids
The genus Cannabis includes C. sativa (marijuana), C. indica, and C. ruderalis. Several biologically active cannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC), cannabichromene, cannabigerol, cannabinol, and cannabidiol are isolated from glands (trichomes) in the flowers of female plants(17). The interest in cannabinoids in ocular physiology began when Hepler and Frank demonstrated in the 1970s that patients who smoked marijuana had a reduction in IOP(18). The mechanism by which this occurs has not been completely elucidated. However, there is some evidence suggesting that it is related to a decrease in blood pressure and/or its effect on CB1 and CB2 receptors in the ciliary body and trabecular meshwork, acting to reduce the production of aqueous humor and increase its drainage(17,19). Smoking marijuana or the oral or intravenous administration of cannabinoids induces an approximately 25% reduction in IOP lasting 3 to 4 h(18,20). Although a reduction in IOP and an absence of major toxic effects after topical use of synthetic cannabinoids such as WIN 55,212-2 has been reported in rats and in patients with glaucoma, its administration as eye drops has not yet shown consistent results(17). Therefore, while there is some evidence supporting the use of cannabinoids to treat elevated IOP, further controlled studies are needed to ensure its safety and efficacy(21).
Apart from the hypotensive ocular effect, cannabinoids also exhibit antioxidant, anti-inflammatory, and neuroprotective activities(17,19). The discovery of an endogenous cannabinoid system in retinal cells of the retina indicates a potential activity of cannabis in retinal tissue homeostasis. Neurodegeneration remains one of the main causes of visual impairment despite the use of inhibitors of vascular endothelium growth factor (VEGF) and anti-inflammatory drugs to treat ischemic retinopathy. There are as yet no effective therapeutic alternatives to ameliorate this condition. However, in vitro and in vivo experimental models of induced retinal excitotoxicity have shown strong evidence that endocannabinoids and synthetic cannabinoids have a neuroprotective effect on various diseases through a direct effect on the CB1 receptor(19).
Euterpe oleracea (Açaí)
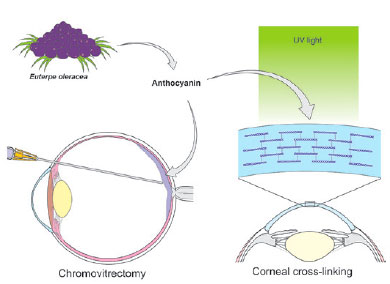
Açaí (E. oleracea) is a palm tree found in northern Brazil. It produces a rounded fruit that has been extensively used in the Brazilian diet and cuisine(22,23). The fruit’s purple color is due to large amounts of anthocyanins, which are flavonoid pigments(22-25). Açaí tincture has been used to dye the posterior hyaloid membrane, facilitating its separation from the internal limiting membrane during chromovitrectomy(25). In 2013, Peris et al. suggested the use of anthocyanins from açaí fruit as a promising alternative for dyeing membranes during vitreoretinal surgery(23). This use was corroborated by Chen et al. using the tincture in human cadaver eyes(25). Caiado et al. studied the toxicity of three concentrations of açaí tincture in chromovitrectomy in rabbit eyes, and suggested that a 10% or 25% might be safe and effective for use in human eyes(24). Addressing potential uses in corneal disease, Bersanetti et al. demonstrated that açaí extract was effective in corneal cross-linking in rabbit corneas (Figure 4)(22).
Curcumin
Curcumin (diferuloylmethane) is a water-insoluble orange powder extracted from the rhizome of long curcumin. It is widely used in Indian cuisine but has also been shown to possess anti-inflammatory and antitumor properties(26). In vitro studies have shown that curcumin reduces cytokine production in cell culture models designed to simulate dry eye conditions(27). In rats, Mrudula et al. demonstrated that a diet enriched with 0.01% curcumin or 0.5% long curcumin reduced VEGF expression in an induced diabetic retinopathy model(28). Clinical studies have reported a reduction in relapse rates of chronic uveitis and improvement of idiopathic inflammatory orbital pseudotumors in patients treated with oral curcumin(29,30).
Gingko biloba
G. biloba leaf extract has been used for hundreds of years by Japanese, Chinese, and Korean people to treat circulatory disorders, asthma, and vertigo(31). The main chemical entities present in G. biloba leaf extract, EGb761 and LI 1370, are responsible for its neuroprotective effect(31). G. biloba has been shown to increase ocular blood flow velocity in healthy adult volunteers, suggesting its potential to improve circulation to the optic nerve head, protecting retinal ganglion cells in patients with glaucoma(32). Evidence from in vitro and clinical studies for the use of G. biloba as an adjuvant in the treatment of normal pressure and high pressure glaucoma that is resistant to other treatments has been reviewed(31,33).
Propolis
Propolis is a resinous substance produced by the interaction of honey bees’ saliva with plant resins, beeswax, and pollen collected from flora(34). Among its biologically-active compounds, caffeic acid phenethyl ester (CAPE) has shown promising anti-inflammatory, immunomodulatory, and antibacterial activity(34-36). Previous reports have demonstrated the beneficial role of CAPE in ocular tissues, whether in cell cultures or animal models(34-39). Totan et al. demonstrated that instillation of CAPE solution in rat corneas has an effect similar effect to that of topical dexamethasone in inhibiting neovascularization(39). Doganay et al. reported that subcutaneously-injected CAPE suppressed the formation of selenite-induced cataract in rats(40). In addition, CAPE has shown promising anti-inflammatory effects in bacterial endophthalmitis and lipopolysaccharide-induced uveitis in rabbits(35,37). Turkos et al. suggested an inhibitory effect of CAPE on proliferative vitreoretinopathy in rabbits(39), while Shi et al. demonstrated the antioxidant properties of CAPE as well as its ability to reduce apoptosis in a rat model of retinal injury (41).
Linum usitatissimum
L. usitatissimum, known in Brazil as linhaça, is a small plant belonging to the linseed family. Its oilseeds, commonly called flaxseeds in English, are rich in essential fatty acids such as omega 6 (γ-linoleic acid) and omega 3 (α-linoleic acid)(42). Pinheiro et al. demonstrated the benefits of L. usitatissimum in reducing ocular inflammation in a controlled study of patients with Sjögren’s syndrome treated daily with 1 g or 2 g of flaxseed oil vs. those receiving a placebo(42).
Heliotropium indicum Linn
The extract of H. indicum Linn (in the Boraginaceae plant family) is widely used in Ghana and other African countries as a natural remedy for various diseases(43,44). Several pharmacologically active alkaloids have been isolated from H. indicum such as indicine, acetyl-indicine, indicinine-N-oxide, heleurine, heliotrine, supine, supinidine, and lindelofidine(44). In 2015, Kyei et al. investigated the activity of an aqueous extract of H. indicum in rabbits’ eyes and identified a significant hypotensive effect in addition to anti-oxidant and neuroprotective effects(45). In 2016, the same authors also demonstrated that oral administration of H. indicum extract in rabbits with lipopolysaccharide-induced uveitis has an anti-inflammatory effect, which they attributed to the extract’s ability to significantly reduce levels of mediators of the inflammatory cascade(46). In the same year, Kyei et al. reported a delay in galactose-induced cataractogenesis in rats treated with an aqueous extract of H. indicum(47).
Carotenoids
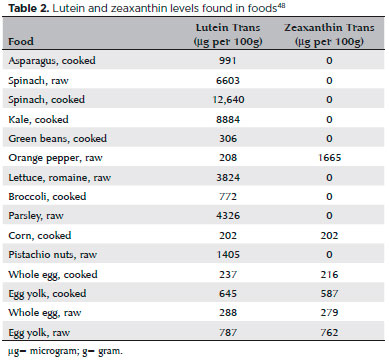
Xanthophylls (lutein and zeaxanthin) are antioxidant carotenoids present in retinal macular pigments(48). These pigments are responsible for protecting the retina against harmful photochemical reactions and for delaying the progression of age-related macular degeneration (AMD), one of the leading causes of blindness in older adults(48,49). Spinach, kale, and orange pepper are the major sources of xanthophylls (Table 2)(48,49). Cohort studies and clinical trials have been carried out to establish the role of these carotenoids in both preventing and delaying the progression of AMD(50,51). Other reports have shown that daily supplementation with β-carotene (15 mg), vitamin C (500 mg), vitamin E (400 UI), zinc (80 mg), and copper (2 mg) decreases the progression to more advanced forms of AMD by 25%, as confirmed by Age-Related Eye Disease Study 1 (AREDS 1)(50). However, β-carotene was associated with a risk of lung cancer in ex-smokers, so it was replaced in AREDS 2 with 10 mg of lutein and 2 mg of zeaxanthin. The new formulation was also able to reduce progression of AMD to more advanced forms (51).
Scutellaria baicalensis Georgi
S. baicalensis Georgi is a plant rich in flavonoids that has been widely used as a medicinal plant in Asian countries such as China. Several bioactive flavonoids, including baicalein (5,6,7-trihydroxyflavone), baicalin (5,6-dihydroxy-7-O-glucuronide), and wogonin (5,7-dihydroxy-8-methoxyflavone) have been extracted from its roots(52). These compounds reportedly have anti-inflammatory, antioxidant, and antiangiogenic properties when applied to ocular tissues(53-56). Hurst and Bazan demonstrated that baicalein reduced the production of platelet-activating factor, a substance responsible for delayed corneal healing in bovine eyes(53). In a rat cataract model, Li et al. demonstrated the inhibitory activity of baicalein and baicalin on the enzyme aldose reductase, which plays a role in cataract formation(54). In a rabbit uveitis model, intravenous injections of baicalein, baicalin, and wogonin as well as ophthalmic instillations of baicalein and baicalin reduced the inflammatory reaction in the anterior chamber of the eye(55,56). The benefits of flavonoids on the retina seem to relate to their antilipoxygenase activity in the inflammatory phase of AMD as well as to protective effects on several layers of the retina(57).
Squalamine
Squalamine lactate (Evizon; Genaera, Plymouth Meeting, PA, USA) is a biologically active amino sterol extracted from the cartilage of the dogfish shark. It has shown promising antiangiogenic properties suitable for the treatment of neovascular AMD and diabetic macular edema(58,59). Its mechanism of action involves the blocking of an endosomal isoform of the sodium pump, therefore disrupting ionic transport and altering endothelial metabolism, with the end result of inhibiting neovascularization(58,60). Squalamine may also block the expression of VEGF when interacting with calmudulin(58,60). Unlike most anti-VEGF therapies, squalamine is administered intravenously and is ineffective intravitreally(58,60). Ciulla et al. reported that squalamine partially reduced laser-induced development of choroidal neovascularization membranes in rat retinas(61). In another animal model of retinopathy designed by Higgins et al., squalamine significantly reduced retinal necrosis(62).
Hyaluronic acid
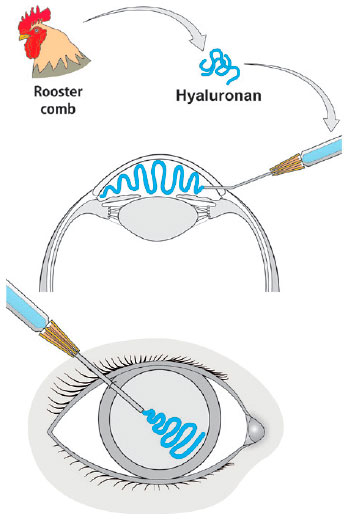
The isolation of uronic acid from the bovine vitreous in 1934 by Karl Meyer and John Palmer gave birth to the term hyaluronic acid (HA) from hyaloid (vitreous) and uronic acid(63,64). HA was later found to be present in the cornea, iris, and crystalline lens, as well as in the extracellular matrix of several tissues(65,66). In spite of its natural origin, the initial extraction of HA from bovine eyes or rooster combs was an expensive and laborious process. Contamination with foreign proteins caused allergic reactions, toxicity, and even anaphylaxis. A larger scale, less expensive method was developed to produce highly pure HA by bacteria such as Streptococcus equi and S. zooepidemicus(64-66). The first product tested for ocular toxicity, immunogenicity, and metabolism was Healon (avg. MW 3 million, 1%, manufactured by Biotrics, Inc., Arlington, MA, USA), which was injected into the eye chambers of monkeys(66). The term viscosurgery was first introduced in the 1980s. Healon was injected to maintain the anterior chamber and protect the endothelium during cataract surgery with intraocular lens implantation (Figure 5)(66-69). Due to its beneficial properties, HA has been successfully used in other ocular surgical procedures. It is also an adjunct therapy for dry-eye syndrome or scarring of the eye surface and is used as a drug delivery system(70-73).
Chitosan
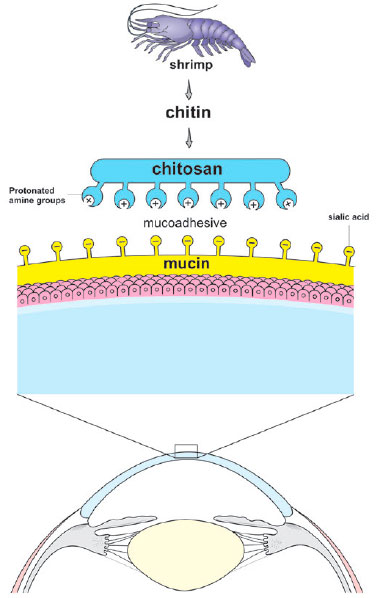
The observation that the ocular mucosal surface has a negative electrical charge due to the presence of sialic acid in the mucin layer has led to consideration of adhering positively-charged polymers to the cornea’s surface(74-79). Such a cationic polymer could deliver drugs to the cornea by adhering to it(74). Chitosan was a good candidate for this use because of its biocompatibility, lack of toxicity, antimicrobial activity, wound-healing properties, and mucoadhesive character (Figure 6)(74,75). This natural polycationic polymer is obtained by the deacetylation of chitin, which is extracted from the exoskeleton of crustaceans. It is the second most abundant polysaccharide in nature, after cellulose(75,80). Its protonated amine groups allow chitosan to interact with negative charges on the surface of the cornea and conjunctiva and therefore play an excellent role in ocular drug delivery(74,75). Whether in the form of gels, implants, or micro- or nanoparticles, chitosan represents one of the main lines of recent research in the delivery of ocular drugs(74,75).
Lycium barbarum (goji berry or wolfberry)
Goji berry or wolfberry is a fruit extracted from L. barbarum or L. chinense that has been used for centuries in traditional Chinese medicine for the treatment of eye diseases and hepatic disorders. It has antioxidant, neuroprotective, anti-inflammatory, and immunomodulatory properties(81,82). These have been attributed to the presence of polysaccharides, which constitute about 40% of the composition of L. barbarum(81). Studies in rats have demonstrated a neuroprotective effect after ingestion of L. barbarum extract in experimental models of retinal ischemia and optic nerve section(81,83,84). The substance has also shown protection against oxidative and apoptotic effects in cultures of human lens epithelial cells(82).
Vaccinium myrtillus (bilberry)
Bilberry is a rounded purple fruit produced by a shrub of the Ericaceae family found in the mountainous regions of Europe and the northern United States(85). Anthocyanosides present in extracts of V. myrtillus have antioxidant, neocollagenesis, and anticoagulant properties(86). Bilberry extract improves night vision, promoting greater adaptation to the dark and faster restoration of visual acuity after exposure to glare(87). In addition, it has been reported that fermented bilberry extracts inhibited the development of form-deprivation myopia induced in guinea pigs(88). In a clinical study, Bravetti et al. demonstrated that 180 mg twice a day of a 25% anthocyanoside extract delayed the progression of senile cortical cataract(89). Riva et al. demonstrated in a placebo-controlled trial that oral administration of bilberry extract increased tear production and improved dry eye symptoms, which was attributed by the authors to its antioxidant properties(90). Yao et al. reported that the use of 100 and 200 mg/kg/day of bilberry extract for five days reduced inflammation in endotoxin-induced uveitis in mice(91). Other in vitro and in vivo studies have reported antioxidant, anti-inflammatory and antiapoptotic effects of V. myrtillus in a visible light-induced retinal degeneration model(92,93).
REINTRODUCTION OF AN OLD TREATMENT IN OPHTHALMOLOGY
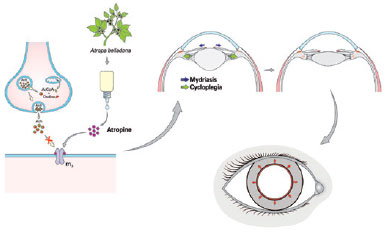
Atropine is an alkaloid extracted from A. belladonna. It has been marketed in sulfate form as eye drops in concentrations of 0.5% and 1% to achieve mydriasis and cycloplegia(1), respectively. The compound is a competitive antagonist of acetylcholine in the muscarinic receptors present in the sphincter of the iris and in the ciliary muscle(1,94). The marked increase in the prevalence of high myopia observed in the past three decades, especially in Asia, has motivated the search for pharmacologic agents to address the issue(94-97). Johannes Kepler postulated the accommodative theory as a cause of progressive myopia, a concept that has brought back the use of atropine(98). In 2006, the clinical trial Atropine in the Treatment of Myopia demonstrated that atropine eye drops slowed the progression of myopia in Asian children(99). In 2016, Chia et al. reported that a lower daily dose of 0.01% atropine eye drops delayed the progression of myopia with less rebound and side effects compared with commonly-used larger doses(100). Other already established molecules have been chemically modified to modulate their clinical efficacy, route of administration, and mechanisms of action in an attempt to solve problems such as the high prevalence of side effects.
FUTURE PERSPECTIVES IN CLINICAL RESEARCH
Since the use of physostigmine as the first herbal product having an application in ophthalmology, nature remains an important source of substances with proven benefits to ocular health. However, while there has been an exponential increase in the number of natural compounds reputed to have ocular activity, most have not been sufficiently studied to prove their safety and efficacy. Therefore, scientific support is lacking, despite the promotion of a growing number of such compounds, including those for which patents are pending.
On the other hand, continued research may enhance the ability to synthesize new compounds based on nature-derived prototypes, giving rise to new pharmacologic options with additional properties. In addition, already-established molecules may be refined to optimize their pharmacokinetics or improve drug delivery systems, ultimately leading to therapies with fewer side effects and greater efficacy.
REFERENCES
1. Duncan G, Collison DJ. Role of the non-neuronal cholinergic system in the eye: A review. Life Sci. 2003;72(18-19):2013-9.
2. Ruetsch YA, Boni T, Borgeat A. From cocaine to ropivacaine: The history of local anesthetic drugs. Curr Top Med Chem. 2001;1(3): 175-82.
3. Orhan IE, Orhan G, Gurkas E. An overview on natural cholinesterase inhibitors--a multi-targeted drug class--and their mass production. Mini Rev Med Chem. 2011;11(10):836-42.
4. Pinho BR, Ferreres F, Valentao P, Andrade PB. Nature as a source of metabolites with cholinesterase-inhibitory activity: An approach to Alzheimer’s disease treatment. J Pharm Pharmacol. 2013;65(12): 1681-700.
5. Realini T. A history of glaucoma pharmacology. Optom Vis Sci. 2011; 88(1):36-8.
6. Markel H. Uber coca: Sigmund freud, carl koller, and cocaine. JAMA. 2011;305(13):1360-1.
7. dos Reis A, Jr. Sigmund freud (1856-1939) and karl koller (1857- 1944) and the discovery of local anesthesia. Rev Bras Anestesiol. 2009;59(2):244-57.
8. Oeppen RS. Discovery of the first local anaesthetic--carl koller (1857-1944). Br J Oral Maxillofac Surg. 2003;41(4):243.
9. Curto EM, Labelle A, Chandler HL. Aloe vera: An in vitro study of effects on corneal wound closure and collagenase activity. Vet Ophthalmol. 2014;17(6):403-10.
10. Wozniak A, Paduch R. Aloe vera extract activity on human corneal cells. Pharm Biol. 2012;50(2):147-54.
11. Atiba A, Wasfy T, Abdo W, Ghoneim A, Kamal T, Shukry M. Aloe vera gel facilitates re-epithelialization of corneal alkali burn in normal and diabetic rats. Clin Ophthalmol. 2015;9:2019-26.
12. Kronschlager M, Lofgren S, Yu Z, Talebizadeh N, Varma SD, Soderberg P. Caffeine eye drops protect against UV-B cataract. Exp Eye Res. 2013;113:26-31.
13. Varma SD, Hegde KR, Kovtun S. UV-B-induced damage to the lens in vitro: Prevention by caffeine. J Ocul Pharmacol Ther. 2008; 24(5):439-44.
14. Varma SD, Kovtun S, Hegde K. Effectiveness of topical caffeine in cataract prevention: Studies with galactose cataract. Mol Vis. 2010;16:2626-33.
15. Zhang S, Zhou R, Li B, Li H, Wang Y, Gu X, et al. Caffeine preferentially protects against oxygen-induced retinopathy. FASEB J. 2017;31(8):3334-48.
16. Chen JF, Zhang S, Zhou R, Lin Z, Cai X, Lin J, et al. Adenosine receptors and caffeine in retinopathy of prematurity. Mol Aspects Med. 2017;55:118-25.
17. Panahi Y, Manayi A, Nikan M, Vazirian M. The arguments for and against cannabinoids application in glaucomatous retinopathy. Biomed Pharmacother. 2017;86:620-7.
18. Hepler RS, Frank IR. Marihuana smoking and intraocular pressure. JAMA. 1971;217(10):1392.
19. Kokona D, Georgiou PC, Kounenidakis M, Kiagiadaki F, Thermos K. Endogenous and synthetic cannabinoids as therapeutics in retinal disease. Neural Plast. 2016;2016:8373020.
20. Sun X, Xu CS, Chadha N, Chen A, Liu J. Marijuana for glaucoma: A recipe for disaster or treatment? Yale J Biol Med. 2015;88(3):265-9.
21. Novack GD. Cannabinoids for treatment of glaucoma. Curr Opin Ophthalmol. 2016;27(2):146-50.
22. Bersanetti PA, Bueno TL, Morandim-Giannetti AA, Nogueira RF, Matos JR, Schor P. Characterization of rabbit corneas subjected to stromal stiffening by the acai extract (euterpe oleracea). Curr Eye Res. 2017;42(4):528-33.
23. Peris CS, Badaro E, Ferreira MA, Lima-Filho AA, Ferreira EL, Maia A, et al. Color variation assay of the anthocyanins from Açai Fruit (Euterpe oleracea): a potential new dye for vitreoretinal surgery. J Ocul Pharmacol Ther. 2013;29(8):746-53.
24. Caiado RR, Peris CS, Lima-Filho AA, Urushima JG, Novais E, Badaró E, et al. Retinal toxicity of acai fruit (euterpe oleracea) dye concentrations in rabbits: basic principles of a new dye for chromovitrectomy in humans. Curr Eye Res. 2017;42(8):1185-93.
25. Chen J, Ferreira MA, Farah ME, de Carvalho AM, Alves Ferreira RE, de Moraes Filho MN, et al. Posterior hyaloid detachment and internal limiting membrane peeling assisted by anthocyanins from acai fruit (Euterpe oleracea) and 10 other natural vital dyes: experimental study in cadaveric eyes. Retina. 2013;33(1):89-96.
26. Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014;80(4):249-54.
27. Chen M, Hu DN, Pan Z, Lu CW, Xue CY, Aass I. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 2010;90(3): 437-43.
28. Mrudula T, Suryanarayana P, Srinivas PN, Reddy GB. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem Biophys Res Commun. 2007;361(2):528-32.
29. Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13(4):318-22.
30. Lal B, Kapoor AK, Agrawal PK, Asthana OP, Srimal RC. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res. 2000;14(6):443-7.
31. Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: An adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis. 2012;18:390-402.
32. Chung HS, Harris A, Kristinsson JK, Ciulla TA, Kagemann C, Ritch R. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999;15(3):233-40.
33. Hirooka K, Tokuda M, Miyamoto O, Itano T, Baba T, Shiraga F. The ginkgo biloba extract (EGb 761) provides a neuroprotective effect on retinal ganglion cells in a rat model of chronic glaucoma. Curr Eye Res. 2004;28(3):153-7.
34. Akyol S, Ugurcu V, Balci M, Gurel A, Erden G, Cakmak O, et al. Caffeic acid phenethyl ester: its protective role against certain major eye diseases. J Ocul Pharmacol Ther. 2014;30(9):700-8.
35. Yildirim O, Yilmaz A, Oz O, Vatansever H, Cinel L, Aslan G, et al. Effect of caffeic acid phenethyl ester on treatment of experimentally induced methicillin-resistant Staphylococcus epidermidis endophthalmitis in a rabbit model. Cell Biochem Funct. 2007;25(6):693-700.
36. Paeng SH, Jung WK, Park WS, Lee DS, Kim GY, Choi YH, et al. Caffeic acid phenethyl ester reduces the secretion of vascular endothelial growth factor through the inhibition of the ROS, PI3K and HIF-1α signaling pathways in human retinal pigment epithelial cells under hypoxic conditions. Int J Mol Med. 2015;35(5):1419-26.
37. Yilmaz A, Yildirim O, Tamer L, Oz O, Cinel L, Vatansever, et al. Effects of caffeic acid phenethyl ester on endotoxin-induced uveitis in rats. Curr Eye Res. 2005;30(9):755-62.
38. Turkoz Y, Er H, Borazan M, Yilmaz H, Mizrak B, Parlakpinar H, et al. Use of caffeic acid phenethyl ester and cortisone may prevent proliferative vitreoretinopathy. Mediators Inflamm. 2004;13(2):127-30.
39. Totan Y, Aydin E, Cekiç O, Cihan Dağloğlu M, Borazan M, Dağlioğlu K. et al. Effect of caffeic acid phenethyl ester on corneal neovascularization in rats. Curr Eye Res. 2001;23(4):291-7.
40. Doganay S, Turkoz Y, Evereklioglu C, Er H, Bozaran M, Ozerol E. Use of caffeic acid phenethyl ester to prevent sodium-selenite-induced cataract in rat eyes. J Cataract Refract Surg. 2002;28(8):1457-62.
41. Shi Y, Wu X, Gong Y, Qiu Y, Zhang H, Huang Z, et al. Protective effects of caffeic acid phenethyl ester on retinal ischemia/reperfusion injury in rats. Curr Eye Res. 2010 Oct;35(10):930-7.
42. Pinheiro MN, Jr, dos Santos PM, dos Santos RC, Barros Jde N, Passos LF, Cardoso Neto J. Oral flaxseed oil (linum usitatissimum) in the treatment for dry-eye sjogren’s syndrome patients. Arq Bras Oftalmol. 2007;70(4):649-55.
43. Kyei S, Koffuor GA, Ramkissoon P, Owusu-Afriyie O. Anti-glaucoma potential of heliotropium indicum linn in experimentally-induced glaucoma. Eye Vis (Lond). 2015;2:16-015-0027-1. eCollection 2015.
44. Kyei S, Koffuor GA, Ramkissoon P, Ameyaw EO, Asiamah EA. Anti-inflammatory effect of heliotropium indicum linn on lipopolysaccharide- induced uveitis in new zealand white rabbits. Int J Ophthalmol. 2016;9(4):528-35.
45. Kyei S, Koffuor GA, Ramkissoon P, Abu EK, Sarpong JF. Anti-cataract potential of heliotropium indicum linn on galactose-induced cataract in sprague-dawley rats. Curr Eye Res. 2017;42(3):394-401.
46. Eisenhauer B, Natoli S, Liew G, Flood VM. Lutein and zeaxanthin- food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients. 2017;9(2):10.3390/ nu9020120.
47. Manikandan R, Thiagarajan R, Goutham G, Arumugam M, Beulaja M, Rastrelli L, et al. Zeaxanthin and ocular health, from bench to bedside. Fitoterapia. 2016;109:58-66.
48. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-36.
49. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-15.
50. Xiao JR, Do CW, To CH. Potential therapeutic effects of baicalein, baicalin, and wogonin in ocular disorders. J Ocul Pharmacol Ther. 2014;30(8):605-14.
51. Hurst JS, Bazan HE. The sensitivity of bovine corneal epithelial lyso-PAF acetyltransferase to cyclooxygenase and lipoxygenase inhibitors is independent of arachidonate metabolites. J Ocul Pharmacol Ther. 1997;13(5):415-26.
52. Li SZ, Mao WS, Du XY, Liang SW, Hu BR, Ma YQ. Inhibition of rat lens aldose reductase by flavonoids-matteucinol and baicalein. Yan Ke Xue Bao. 1987;3(2):93-4, 137.
53. Nagaki Y, Hayasaka S, Kadoi C, Nakamura N, Hayasaka Y. Effects of scutellariae radix extract and its components (baicalein, baicalin, and wogonin) on the experimental elevation of aqueous flare in pigmented rabbits. Jpn J Ophthalmol. 2001;45(3):216-20.
54. Nagaki Y, Hayasaka S, Zhang XY, Hayasaka Y, Nakamura N, Terasawa K. Effects of topical instillation of traditional herbal medicines, herbal extracts, and their components on prostaglandin E2-induced aqueous flare elevation in pigmented rabbits. Jpn J Ophthalmol. 2003;47(3):249-53.
55. Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Invest Ophthalmol Vis Sci. 2005;46(12):4796-803.
56. Emerson MV, Lauer AK. Emerging therapies for the treatment of neovascular age-related macular degeneration and diabetic macular edema. BioDrugs. 2007;21(4):245-57.
57. Bhargava P, Marshall JL, Dahut W, Rizvi N, Trocky N, Williams JI, et al. A phase I and pharmacokinetic study of squalamine, a novel antiangiogenic agent, in patients with advanced cancers. Clin Cancer Res. 2001;7(12):3912-9.
58. Michels S, Schmidt-Erfurth U, Rosenfeld PJ. Promising new treatments for neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2006;15(7):779-93.
59. Ciulla TA, Criswell MH, Danis RP, Williams JI, McLane MP, Holroyd KJ. Squalamine lactate reduces choroidal neovascularization in a laser-injury model in the rat. Retina. 2003;23(6):808-14.
60. Higgins RD, Sanders RJ, Yan Y, Zasloff M, Williams JI. Squalamine improves retinal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(6):1507-12.
61. Liu L, Liu Y, Li J, Du G, Chen J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb Cell Fact. 2011;10:99-2859-10-99.
62. Lapcik L Jr and L, Lapcik L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: Preparation, structure, properties, and applications. Chem Rev. 1998;98(8):2663-84.
63. Guter M, Breunig M. Hyaluronan as a promising excipient for ocular drug delivery. Eur J Pharm Biopharm. 2017;113:34-49.
64. Balazs EA. Hyaluronan as an ophthalmic viscoelastic device. Curr Pharm Biotechnol. 2008;9(4):236-8.
65. Gundorova RA, Khoroshilova IP, Brikman IV, Ibadova SI, Ilatovskaia LV. A new agent for viscosurgery of the eye. Oftalmol Zh. 1987;(7): 427-9.
66. Hessburg PC. The cost of materials used in viscosurgery. Ophthalmology. 1986;93(2):276.
67. Van Oye R. Viscosurgery. Bull Soc Belge Ophtalmol. 1982;201:47-50.
68. Stuart JC, Linn JG. Dilute sodium hyaluronate (healon) in the treatment of ocular surface disorders. Ann Ophthalmol. 1985;17(3):190-2.
69. Prabhasawat P, Tesavibul N, Kasetsuwan N. Performance profile of sodium hyaluronate in patients with lipid tear deficiency: Randomised, double-blind, controlled, exploratory study. Br J Ophthalmol. 2007;91(1):47-50.
70. Ibrahim HK, El-Leithy IS, Makky AA. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/ prednisolone bitherapy. Mol Pharm. 2010;7(2):576-85.
71. Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010;31(9):2555-64.
72. Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Chitosan and its role in ocular therapeutics. Mini Rev Med Chem. 2009;9(14):1639-47.
73. Basaran E, Yazan Y. Ocular application of chitosan. Expert Opin Drug Deliv. 2012;9(6):701-12.
74. Araujo J, Gonzalez E, Egea MA, Garcia ML, Souto EB. Nanomedicines for ocular NSAIDs: Safety on drug delivery. Nanomedicine. 2009;5(4):394-401.
75. de Campos AM, Diebold Y, Carvalho EL, Sanchez A, Alonso MJ. Chitosan nanoparticles as new ocular drug delivery systems: In vitro stability, in vivo fate, and cellular toxicity. Pharm Res. 2004; 21(5):803-10.
76. Gong K, Darr JA, Rehman IU. Supercritical fluid assisted impregnation of indomethacin into chitosan thermosets for controlled release applications. Int J Pharm. 2006;315(1-2):93-8.
77. Prabaharan M, Mano JF. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005;12(1):41-57.
78. Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100(1):5-28.
79. Wang H, Lau BW, Wang NL, Wang SY, Lu QJ, Chang RC, et al. Lycium barbarum polysaccharides promotes in vivo proliferation of adult rat retinal progenitor cells. Neural Regen Res. 2015;10(12): 1976-81.
80. Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G, et al. Lycium barbarum polysaccharides protect human lens epithelial cells against oxidative stress-induced apoptosis and senescence. PLoS One. 2014;9(10):e110275.
81. Yang D, So KF, Lo AC. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin Exp Ophthalmol. 2017.
82. Li HY, Ruan YW, Kau PW, Chiu K, Chang RC, Chan HH, et al. Effect of Lycium barbarum (Wolfberry) on alleviating axonal degeneration after partial optic nerve transection. Cell Transplant. 2015;24(3):403-17.
83. Song J, Li Y, Ge J, Duan Y, Sze SC, Tong Y, et al. Protective effect of bilberry (Vaccinium myrtillus L.) extracts on cultured human corneal limbal epithelial cells (HCLEC). Phytother Res. 2010;24(4):520-4.
84. Jakobsdottir G, Nilsson U, Blanco N, Sterner O, Nyman M. Effects of soluble and insoluble fractions from bilberries, black currants, and raspberries on short-chain fatty acid formation, anthocyanin excretion, and cholesterol in rats. J Agric Food Chem. 2014;62(19): 4359-68.
85. Kamiya K, Kobashi H, Fujiwara K, Ando W, Shimizu K. Effect of fermented bilberry extracts on visual outcomes in eyes with myopia: A prospective, randomized, placebo-controlled study. J Ocul Pharmacol Ther. 2013;29(3):356-9.
86. Deng HW, Tian Y, Zhou XJ, Zhang XM, Meng J. Effect of bilberry extract on development of form-deprivation myopia in the guinea pig. J Ocul Pharmacol Ther. 2016;32(4):196-202.
87. Chu W, Cheung SCM, Lau RAW, Benzie IFF. Bilberry (vaccinium myrtillus L.). In: Benzie IFF, Wachtel-Galor S, eds. Herbal medicine: Biomolecular and clinical aspects. 2nd ed. Boca Raton (FL): by Taylor and Francis Group, LLC; 2011. NBK92770 [bookaccession].
88. Riva A, Togni S, Franceschi F, Kawada S, Inaba Y, Eggenhoffner R, et al. The effect of a natural, standardized bilberry extract (Mirtoselect®) in dry eye: a randomized, double blinded, placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2017;21(10):2518-25.
89. Yao N, Lan F, He RR, Kurihara H. Protective effects of bilberry (vaccinium myrtillus L.) extract against endotoxin-induced uveitis in mice. J Agric Food Chem. 2010;58(8):4731-6.
90. Matsunaga N, Imai S, Inokuchi Y, Shimazawa M, Yokota S, Araki Y, et al. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol Nutr Food Res. 2009;53(7):869-77.
91. Ogawa K, Tsuruma K, Tanaka J, Kakino M, Kobayashi S, Shimazawa M, et al. The protective effects of bilberry and lingonberry extracts against UV light-induced retinal photoreceptor cell damage in vitro. J Agric Food Chem. 2013;61(43):10345-53.
92. Pineles SL, Kraker RT, VanderVeen DK, Hutchinson AK, Galvin JA, Wilson LB, et al. Atropine for the prevention of myopia progression in children: A report by the american academy of ophthalmology. Ophthalmology. 2017;124(12):1857-66.
93. Wang TJ, Chiang TH, Wang TH, Lin LL, Shih YF. Changes of the ocular refraction among freshmen in national taiwan university between 1988 and 2005. Eye (Lond). 2009;23(5):1168-9.
94. Galvis V, Tello A, Parra MM, Merayo-Lloves J, Larrea J, Julian Rodriguez C, et al. Topical atropine in the control of myopia. Med Hypothesis Discov Innov Ophthalmol. 2016;5(3):78-88.
95. Saw SM, Tong L, Chua WH, Chia KS, Koh D, Tan DT, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46(1):51-7.
96. Mark HH. Johannes Kepler on the eye and vision. Am J Ophthalmol. 1971;72(5):869-78.
97. Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-91.
98. Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: Myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391-9.
Submitted for publication:
November 13, 2017.
Accepted for publication:
March 13, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose