

Danielle Alves Silva1; Gisele Alborgetti Nai2; Rogério Giuffrida1; Marcos Rógério Sgrignoli1; Daniela Rodrigues dos Santos3; Isabela Vasconcelos Donadão3; Felipe Franco Nascimento3; Heloise Rangel Dinallo3; Silvia Franco Andrade1
DOI: 10.5935/0004-2749.20180081
ABSTRACT
Purpose: To compare the efficacy of 0.03% topical tacrolimus in combination with oral omega (ω) 3 with different ratios of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and antioxidants as adjuvant in the treatment of keratoconjunctivitis sicca (KCS) in dogs.
Methods: Forty-five dogs with KCS were evaluated monthly for 6 months. Evaluations included performance of the Schirmer tear, fluorescein, and lissamine green tests. Tear film break-up time (TBUT) was assessed. Conjunctival cytology was evaluated at the beginning, middle, and end of the study. Conjunctiva was biopsied at the beginning and end of the study. Dogs were randomly assigned to one of the three groups (n=15): Group T (topical tacrolimus 0.03%), Group TO (topical tacrolimus + ω-1.5 EPA: 1 DHA), or Group TOA (topical tacrolimus + ω-1 EPA:4.5 DHA + antioxidants).
Results: There was a significant improvement in clinical signs in all groups. TBUT increased throughout treatment in all groups; this effect was most pronounced in Group TO. Cytological analysis performed at the end of the study period, showed decreased levels of lymphocytes, neutrophils, and metaplastic and squamous cells in Groups T, TO, and TOA. Histological analysis performed at the end of the study period showed decreased levels of lymphocytes and neutrophils and increased levels of goblet cells. These effects were most pronounced in Group TO.
Conclusion: Oral treatment with ω-3 containing a higher proportion of EPA than DHA increased the effectiveness of topical tacrolimus 0.03% in the treatment of keratoconjunctivitis sicca in dogs.
Keywords: Keratoconjunctivitis sicca; Tacrolimus; Eicosapentaenoic acid; Docosahexaenoic acids; Antioxidants; Animal; Dogs
RESUMO
Objetivo: Comparar a eficácia do tacrolimus 0,03% tópico associado ao ômega 3 oral, com diferentes proporções de ácido eicosapentaenoico (EPA), ácidos docosa-hexaenoicos (DHA) e antioxidantes, como adjuvante no tratamento de cães acometidos por ceratoconjuntivite seca.
Métodos: Quarenta e cinco cães atendidos no Hospital Veterinário da UNOESTE portadores de ceratoconjuntivite seca foram avaliados mensalmente por 6 meses pelo Teste Lacrimal de Schirmer, Teste de Fluoresceína, Tempo de Ruptura do Filme Lacrimal, Teste de Rosa Bengala, citologia da conjuntiva no início, meio e fim do projeto e biopsia da conjuntiva no início e final do projeto. Os cães foram distribuídos aleatoriamente em 3 grupos (n=15): grupo T (tacrolimus 0,03% tópico), grupo TO (tacrolimus + ômegas 1.5 EPA/1 DHA oral) e grupo TOA (tacrolimus + ômegas 1 EPA/4,5 DHA + antioxidantes oral).
Resultados: Houve uma melhora significativa nos sinais clínicos em ambos os grupos. No tempo de ruptura do filme lacrimal todos os grupos apresentaram aumento no decorrer do tratamento, sendo que o grupo TO foi o que apresentou melhor resultado em todos momentos quando comparado aos demais grupos. Ao final do experimento, os grupos T, TO e TOA apresentaram na análise citológica, diminuição de linfócitos, neutrófilos, células metaplásicas e escamosas, e na análise histopatológica, diminuição de linfócitos e neutrófilos e aumento das células caliciformes, com o grupo TO com melhor desempenho.
Conclusão: O tratamento oral com ω-3 contendo uma maior proporção de EPA do que o DHA aumentou a eficácia do tacrolimus tópico 0,03% no tratamento de ceratoconjuntivite sicca em cães.
Descritores: Ceratoconjuntivite seca; Tacrolimo; Ácido eicosapentaenoico; Ácidos docosa-hexaenoicos; Antioxidantes; Animais; Cães
INTRODUCTION
Keratoconjunctivitis sicca (KCS), also known as dry eye syndrome, is an ocular disease resulting from inflammation of the lacrimal gland and decreased lacrimal film. KCS can occur because of reduced production of the aqueous portion of the tear film (quantitative deficiency) and/or excessive evaporation of the tear film (qualitative deficiency). Both phenomena render the protective function of the tear film deficient. KCS mainly affects structures of the cornea and conjunctiva(1,2). Among the causes of KCS in dogs are racial predisposition, hypothyroidism, medications (atropine, sulfonamides), surgical excision of the third eyelid gland, distemper, and, mainly, autoimmune factors(3-5).
The main clinical signs observed are mucoid or mucopurulent secretion, conjunctival hyperemia, pigmentation, vascularization and opacity of the cornea, blepharitis, blepharospasm, and, in cases of greater severity, presence of corneal ulcer(6).
Tacrolimus (FK 506) is a macrolide antibiotic isolated from Streptomyces tsukubaensis spp that has an immunomodulatory action(4). The effects observed with its use are the combination of local immunosuppression, goblet cell proliferation, and anti-inflammatory effects(7,8).
Recent studies in humans(9-11) and animals(12,13) showed good results in dry eye control with the use of essential fatty acids, ω-3 and ω-6, due to their ability to produce anti-inflammatory mediators.
Fish oil (FO) from cold-water fish (e.g., salmon, tuna, herring)(9) is an important source of ω-3. Ingestion of marine plants that synthesize ω-3 results in the formation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Other essential oils, such as flaxseed oil, require conversion of β-linolenic acid (ALA) to EPA and DHA(10,11).
EPA plays an important anti-inflammatory role, whereas DHA contributes to function and development of the retina(14) and brain(15). In addition to EPA and DHA, ω-3 is a precursor of lipid mediators, such as resolvins and protectins, which have anti-inflammatory and immunomodulatory actions(16,17).
Several studies have shown that nutritional supplementation with antioxidants, vitamins (A, C, and E), and minerals improves visual function(18). Antioxidants assist in the anti-inflammatory response, corneal healing, and tear film stability(19).
The objective of the study was to compare the efficacy of 0.03% topical tacrolimus in combination with oral ω-3 with different ratios of EPA, DHA, and antioxidants as an adjuvant in the treatment of KCS in dogs.
METHODS
Animals
The study was conducted according to the standards of animal experimentation of the UNOESTE Ethical Committee on Animal Use (protocol #2939) and the statement from the Association for Research in Vision and Ophthalmology for the use of animals in ophthalmic and visual research.
We evaluated 45 dogs seen at the Veterinary Hospital of UNOESTE over a 6-month period. Inclusion in the study was not based on any preference in terms of breed or sex. Dogs were diagnosed with KCS and registered under an authorization term (Informed Consent Term). The animals were included in the experiment by observing clinical ophthalmic signs compatible with KCS (ocular secretion, conjunctivitis, corneal opacity, and pigmentation) with a slit lamp (Kowa SL-15, Japan) as well as SST ≤10 mm/min and/or TBUT ≤10 s.
Groups
After the diagnosis of KCS, the dogs were randomly divided in a double-blind study into three treatment groups: T group (n=15): tacrolimus 0.03% eye drops (Eye Pharma Laboratory, São Paulo, Brazil), one drop, 2×/day, topical, in both eyes for 6 months; TO group (n=15) tacrolimus eye drops 0.03% (Eye Pharma Laboratory, São Paulo, Brazil), one drop 2×/day, topical in both eyes + oral ω-3 (1.5 EPA: 1 DHA-Ograx®-3, Avert Laboratory, São Paulo, Brazil, 1 capsule of 500 mg/7 kg/day) for 6 months; Group TOA (n=15) tacrolimus eye drops 0.03% (Eye Pharma Laboratory, São Paulo, Brazil), one drop 2×/day, topical in both eyes + oral ω-3 + antioxidants (1 EPA: 4,5 DHA + vitamin E + vitamin C + selenium- Seniox®, Avert Laboratory, São Paulo, Brazil, 1 capsule of 500 mg/10 kg/day), for 6 months.
All groups also received propylene glycol-based lubricant (Systane®, Alcon, São Paulo, Brazil), one drop, both eyes, twice daily for 6 months. If necessary, antibiotic eye drops were used (one drop, four times per day, for 15 days), based on culture and antimicrobial sensitivity from ocular samples secretions of all of the dogs and associated too on the presence of corneal ulcer or clinical signs such conjunctivitis and mucopurulent ocular secretion. Anti-inflammatory eye drops containing diclofenac sodium (Still®, Allergan, São Paulo, Brazil) (one drop, three times per day, for 15 days) were used only in eyes with discomfort and ocular hyperemia without corneal ulcers.
Ophthalmic exams
Monthly ophthalmic and cytological exams were performed, considering zero (T0) the first day of treatment with bilateral KCS diagnosis and the other time points (T1-T6). The cytological examination was performed at moments T0, T3, and T6, and histopathological examination was performed at the time of diagnosis (T0) and at the end of the study (T6).
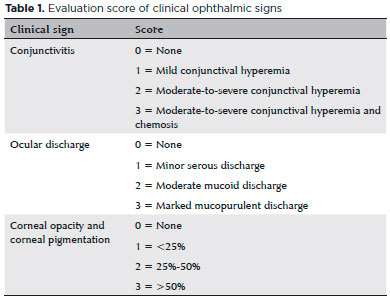
Clinical ophthalmic signs were identified using the portable slit lamp (Kowa, Japan) with or without conjunctivitis, ocular secretion, opacity, and corneal pigmentation according to the scoring described in table 1.
The Schirmer tear test (STT) was performed without anesthetic eye drops to evaluate the quantitative portion of the tear film. A 0.5-cm strip was introduced into the conjunctival sac for 1 min. Results ≤10 mm/min were considered positive(20). Tear film break-up time (TBUT) was used to evaluate the qualitative portion of the tear film. The mean of two consecutive measurements was recorded. After instilling one drop of 1% fluorescein (Allergan, São Paulo, Brazil), the eye was examined under the slit-lamp (Kowa, Japan). Time from the last blink to the appearance of dark spots on the lacrimal film was measured. TBUT values ≤10 s were considered as positive results(21).
After TBUT examination, eyes were washed with physiological saline. The presence or absence of corneal ulcers was determined using the fluorescein test (FT)(20). Ulcers were graded according to severity and extent (0: negative; 1: small superficial ulcer; 2: medium superficial ulcer; 3: extensive superficial ulcer; 4: small stromal ulcer; 5: medium stromal ulcer; 6: extensive stromal ulcer; 7: descemetocele; and 8: keratomalacia or melt ulcer).
Green lissamine test (GLT) strips (Ophthalmos, São Paulo, Brazil) were used to evaluate the presence of devitalized cells(22). The lissamine strip was placed in contact with the tear meniscus at the bottom of the corneal sulcus; measurements were obtained 2-min later. The classification of van Bijsterveld for graduation was used. The palpebral rhyme was divided into three areas: lateral bulbar conjunctiva, cornea, and medial bulbar conjunctiva. In each of these areas, the following graduation was used: 0-absence of staining, 1-fine dots, 2-dots coarse, and 3-plate. The sum of each of these areas determined the final score, which ranged from 0 to 9.
Cytological and histopathological examination
Cytology was evaluated after the eye had been cleaned with physiological saline solution. Cells were obtained from the lower conjunctiva with a sterile swab that had been moistened with physiological solution. Staining was performed with MGG technique (May-Grunwald-Giemsa). Lymphocytes, neutrophils, metaplastic cells, and squamous cells were counted under an optical microscope in 10 fields with a 40× objective lens.
The histopathological examination was performed after instillation of Anestésico® (1% tetracaine hydrochloride + 0.1% phenylephrine hydrochloride, Allergan, São Paulo, Brazil) with a withdrawal of 1-3 mm in the fornix of the medial inferior conjunctiva using tweezers and conjunctive scissors. The histological section was placed in a standardized 1 × 1 cm paper size fixed in formaldehyde and embedded in paraffin (Dynamics Analytical Reagents, São Paulo, Brazil). With the help of a rotating microtome, 5-µm-thick sections of the conjunctiva were obtained, stained with hematoxylin and eosin (HE) (Dolles, São Paulo, Brazil), PAS (Merck, USA), and subsequently evaluated for the following parameters. In HE staining: lymphocyte count, neutrophils, presence of squamous metaplasia, and presence of edema. In PAS counting, the goblet cell density (cells/mm2) under the optical microscope at 40×.
Statistical analysis
Two analyses of variance for Tukey’s sample were used for statistical analysis of results obtained with the STT and TBUT, as well as measurements of goblet cell density and numbers of squamous cells, metaplastic cells, neutrophils, and lymphocytes. For the clinical sign variables FT and GLT, we used Friedman’s nonparametric test to compare moments, while the Kruskall–Wallis test with Dunn contrast was used to compare the groups. A p<0.05 significance level was adopted. The software used for statistical analysis was R, version 3.2.2. (The R Foundation for Statistical Computing, 2015).
RESULTS
In the clinical signs evaluated (Figure 1), all groups showed significant improvement. In the variables of ocular secretion and conjunctivitis, there was total remission in T1 only in Group TO, whereas in Groups T and TOA, remission was found at T3 and T2, respectively. In the variable of opacity, there was total remission in all groups in T2. Regarding corneal pigmentation, the median showed reduction throughout the treatment in all groups.
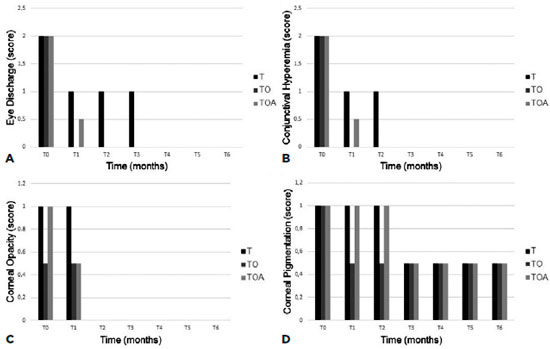
In all three groups, STT values significantly increased from baseline at T1 and all subsequent time-points (p<0.05; Figure 2). At the time T6, the T group presented a significant difference in relation to the other groups. In the TBUT (Figure 2), all groups presented significant differences in relation to the moment T0. However, Group TO presented the best results at all times compared with the other groups.
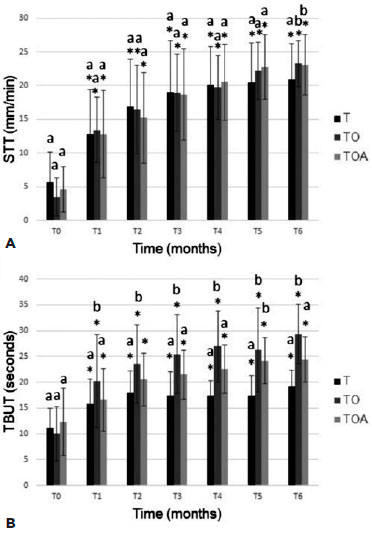
In FT, all groups presented animals with ulcers of varying severity and extension at T0, and all groups showed excellent healing in T1. In the GLT, the performance of all groups was similar, demonstrating improvement in the marking already found in T2.
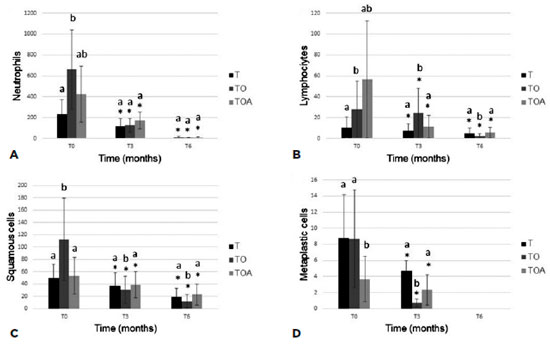
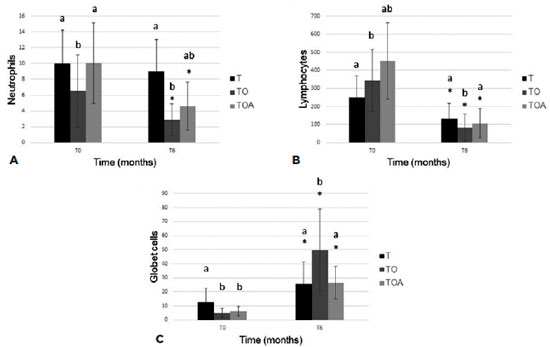
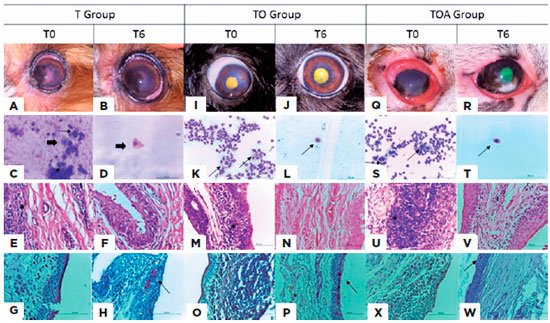
Cytological analysis showed decreased levels of all cell populations examined, but differences at a given time-point were only significant in comparison with baseline levels (Figure 3). In the histopathological study (Figure 4), all groups showed a decrease of inflammatory cells. The goblet cells showed a significance increased (p<0.05) in TO group (49.7 ± 29.4), in relation to other groups, T (25.5 ± 15.6) and TOA (26.5 ± 11.7). Representative images of some eyes and cytological and histopathological results for Groups T, TO, and TOA are presented in figure 5.
DISCUSSION
The use of ω-3 (EPA and DHA) improved the clinical signs of KCS, increasing the STT and TBUT values and promoting resolution of corneal ulcers, because they presented anti-inflammatory characteristics and corroborated the results of other studies(9,10).
In the present study, resolution of ocular secretion, conjunctivitis, and increased TBUT were better in Group TO compared with the T and TOA groups. Both TO and TOA groups received ω supplementation, at varying proportions: Group TO (1.5EPA:1DHA) and the TOA group (1EPA:4.5DHA + antioxidants), in which addition of antioxidants did not seem to have any treatment benefits, had a portion of EPA. According to another study(23), efficacy in treatment for dry eye with use of ω-3 is linked to the dose of ingested ω; however, the results of our study show that effectiveness is related to relative proportions of EPA and DHA. Other studies(19) referred to antioxidants as an adjunct to the inflammatory response, corneal healing, and lacrimal film stability. However, in the present study, this adjunct did not bring additional benefits in the treatment of dry eye in dogs.
According to studies, EPA has a pro-inflammatory effect exerting cellular actions that stimulate the production of collagenase and increase expression of adhesion molecules necessary for leukocyte extravasation. EPA is the precursor of the E series of resolvins, which includes resolvin E1 (RvE1) and resolvin E2 (RvE2)(24-26). Resolvins were first described in connection with the formation of mediator molecules with anti-inflammatory capacity and immunomodulatory properties, including the reduction of proinflammatory leukocytes and cytokine migration, thus leading to a decreased inflammatory response in vivo(27).
DHA is more related to its antioxidant properties and is involved in several cognitive processes while also linked to correct signaling between neurons, in fetal development and retinal function. DHA can also give rise to resolvins D1 (RvD1) and D2 (RvD2), a protectin (or neuroprotetin, when produced by neural tissues) and a maresine, thus having an important anti-inflammatory function in neuronal systems and the formation, development, and functioning of the brain and retina(24,25,27).
In the present study, conjunctival biopsy was performed to assess inflammatory cytology. Neutrophil counts were lower in Group TO compared with other groups at time T6. This effect may be attributable to anti-inflammatory mediator RvE1 (produced by EPA), which increases macrophage phagocytic activity(28).
There was a significant increase in the number of goblet cells from baseline to the end of the study period. This effect was most apparent in Group TO. Similar results were reported previously; one study found an increase in the number of goblet cells after administration of ω-fatty acids for treatment of KCS(9,10).
The results reported above indicate decreased clinical signs and inflammation, as well as increased numbers of goblet cells after treatment with an oral formulation of ω-3 containing a higher proportion of EPA than DHA. This formulation of ω-3 fatty acids enhances the effectiveness of topical tacrolimus in treating canine KCS. We attribute this effect to higher levels of EPA, which is a precursor of anti-inflammatory and immunomodulatory factors such as RvE1.
REFERENCES
1. Ribeiro AP, Brito FL, Martins BC, Mamede F, Laus JL. Qualitative and quantitative tear film abnormalities in dogs. Cienc Rural. 2008; 38(2):568-75.
2. Williams DL. Immunopathogenesis of keratoconjunctivitis sicca in the dog. Vet Clin North Am Small Anim Pract. 2008;38(2):251-68.
3. Gelatt KN. Veterinary Ophthalmology. 3th ed. Pennsylvania: Lippincott Williams & Wilkins; 2005. 594p.
4. Miller PE. Lacrimal system. In: Maggs DJ, Miller PE, Ofri R. Slater’s Fundaments of Veterinary Ophthalmology. 4th ed. St Louis: Elsevier; 2008. p. 157-74.
5. Berdoulay A, English RV, Nadelstein B. Effect of topical 0.02% tacrolimus aqueous suspension on tear production in dogs with keratoconjunctivitis sicca. Vet Ophthalmol. 2005;8(4):225-32.
6. Koch SA, Sykes J. Keratoconjunctivitis sicca. In: Riis RC. Small animal ophthalmology secrets. Philadelphia: Hanley & Belfuscap; 2002. p. 52-60
7. Hendrix VD, Adkins EA, Ward, DA, Stuffle J, Skorobohach B. An investigation comparing the efficacy of topical ocular application of tacrolimus and cyclosporine in dogs. Vet Med Int. 2011; 2011:487592.
8. Moskovici BK, Holzchuh R, Sakassegawa-Naves FE, Hoshino-Ruiz DR, Albers MB, Santo RM, et al. Treatment of Sjögren’s syndrome dry eye using 0.03% tacrolimus eyedrop: Prospective double-blind randomized study. Cont Lens Anterior Eye. 2015;38(5):373-8.
9. Rand AL, Asbell PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22(4):279-82.
10. Jenkins DJ, Josse AR. Fish oil and omega-3 fatty acids. CMAJ. 2008; 178(2):150.
11. Rashid S, Jin Y, Ecoiffer T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):210-25.
12. Neves ML, Yamasaki L, Sanches OC, do Amaral MS, Stevanin H, Giuffrida R, et al. Use of linseed oil to treat experimentally induced keratoconjunctivitis sicca in rabbits. J Ophthalmic Inflamm Infect. 2013;3(1):4.
13. Silva DA, Nai GA, Giuffrida R, Barbero RC, Kuhn JM, da Silva AD, et al. Comparison between fish and linseed oils administered orally for the treatment of experimentally induced keratoconjunctivitis sicca in rabbits. Open Vet J. 2017;7(3):277-85.
14. Cheatham CL, Colombo J, Carlson SE. N-3 fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. Am J Clin Nutr. 2008;83(6 Suppl):1458S-66S.
15. Wurtman RJ. Synapse formation and cognitive brain development: effect of docosahexaenoic acid and other dietary constituents. Metabolism. 2008;57 Suppl 2:S6-10.
16. Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2(3):355-74
17. Serhan, CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirik G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammatory signals. J Exp Med. 2002;196(8):1025-37.
18. Cangemi FE. TOZAL Study: an open case control study of an oral antioxidant and omega-3 supplement for dry AMD. BMC Ophthalmol. 2007;7:3.
19. Gus PI, Belló-klein A, Llesuy S, Quinto GG, Matos GH, Bechara SJ. Tear antioxidante potential in Young adults. Arq Bras Oftalmol. 2006;69(4):565-70.
20. Maggs DJ. Basic diagnostic techniques. In: Maggs DJ, Miller PE, Ofri R, editors. Slatter’s Fundamentals of Veterinary Ophthalmology. St. Louis: Saunders Elsevier; 2008. p. 81-106.
21. Saito A, Kotani T. Estimation of lacrimal level and testing methods on normal beagles. Vet Ophthalmol. 2001;4(1):7-11.
22. Machado LM, Castro RS, Fontes BM. Staining patterns in dry eye syndrome: rose bengal versus lissamine green. Cornea. 2009; 28(7):732-4.
23. Escamilla NE. Omega 3 Y su acción terpéutica em el síndrome de ojo seco. Ciência & Tecnologia Para La Salud Visual y Ocular [Internet]. 2003[cited 2016 jun 21];1(1):91-8. Available from: https://revistas.lasalle.edu.co/index.php/sv/article/view/1946
24. Hodge WG, Schachter HM, Barnes D, Pan Y, Lowcock EC, Zhang L, et al. Efficacy of W-3 fatty acids in preventing age-related macular degeneration-a systematic review. Ophthalmology. 2006;113(7): 1165-72; quiz 1172-3, 1178.
25. Seki H, Sasaki T, Ueda T, Arita M. Resolvins as regulators of the immune system. Sci World J. 2010;10:818-31.
26. Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713-22.
27. Bannenberg GL. Therapeutic applicability of anti-Inflammatory and proresolving polyunsaturated fatty acid-derived lipid mediators. Sci World J. 2010;10:676-712.
28. Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21(12):3162-70.
Submitted for publication:
November 22, 2017.
Accepted for publication:
February 9, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Approved by the following research ethics committee: Ethics Committee on the Use of Animals of UNOESTE (# 2939)