

Luiz Guilherme Azevedo de Freitas1,2; David Leonardo Cruvinel Isaac1,3; Eliana Martins Lima4; Leonardo Gomes Souza4; Murilo Alves Abud1; Ricardo Gomes dos Reis1; William Thomas Tannure1; Marcos Pereira de Ávila1
DOI: 10.5935/0004-2749.20180079
ABSTRACT
Purpose: We aimed to evaluate the safety of single intravitreal injection of each of two concentrations of 0.1 ml of sunitinib (1 and 10 mg/ml), 0.1 ml of a drug-free dispersion containing solid lipid nanoparticles, and 0.1 ml of a drug-free dispersion containing polymeric nanocapsules for analyzing the possible toxic effects using electrophysiology and histology in albino rabbit retina.
Methods: We conducted an experimental controlled study of 20 eyes of albino rabbits. Intravitreal injections of each specific agent were applied to one eye per rabbit in each 5-rabbit group, while the contralateral eyes received no treatment and were used as controls.
Results: We noted no electroretinographic changes in the sunitinib (1 and 10 mg/ml) or in solid lipid nanoparticles groups. However, we observed significant abnormalities in ocular morphology and in the electroretinogram in the nanocapsules group. At the histological level, only the nanocapsules group demonstrated abnormal changes, including severe edema and cytoplasmic vacuole formation.
Conclusions: While nanocapsules intravitreal injections indicated retinal toxic effects, sunitinib and solid lipid nanoparticles intravitreal injections were not toxic to the retina. Our results suggest that a sunitinib preparation with solid lipid nanoparticles for controlled release may offer a significant therapeutic approach for vasoproliferative ocular disease.
Keywords: Pathological neovascularization; Angiogenesis inhibitors; Nanotechnology; Nanoparticles; Intravitreal injections; Rabbits
RESUMO
Objetivos: O presente estudo teve por objetivo avaliar a segurança da injeção intravítrea de 0,1 ml de sunitinibe em duas concentrações (1 mg/ml e 10 mg/ml), 0,1 ml de dispersão contendo nanopartículas lipídicas sólidas sem droga e 0,1 ml de dispersão contendo nanocápsulas poliméricas livre de drogas analisando os possíveis efeitos tóxicos à retina de coelhos albinos detectados pela eletrofisiologia e histologia por microscopia óptica.
Métodos: Um estudo controlado experimental foi realizado com 20 olhos de coelhos albinos. Foram realizadas injeções intravítrea de duas concentrações diferentes de sunitinibe, uma dispersão contendo nanopartículas lipídicas sólidas e uma dispersão contendo nanocápsulas. O olho contralateral não recebeu tratamento e foi utilizado como controle.
Resultados: Não foram observadas alterações eletrorretinográficas nos grupos do sunitinibe (1 mg/ml e 10 mg/ml) e no grupo das nanopartículas lipídicas sólidas. No grupo das nanocápsulas, houve alterações significativas tanto na morfologia, quanto na amplitude e tempo das ondas do eletrorretinograma. Ao estudo histológico, somente o grupo das nanocápsulas apresentou alterações degenerativas (núcleos tumefeitos) com acentuado edema e formação de vacúolos citoplasmáticos, sugerindo toxidade retiniana.
Conclusões: As injeções intravítreas de sunitinibe e nanopartículas lipídicas sólidas não foram tóxicas para a retina. No entanto, nanocápsulas mostraram ser tóxicas para a retina. Sendo assim, a possibilidade de poder combinar o potencial de uma droga que possui a capacidade de inibir duas importantes vias da angiogênese, às vantagens de liberação controlada das nanopartículas lipídicas sólidas, pode ser um importante recurso terapêutico para doenças vasoproliferativas oculares.
Descritores: Neovascularização patológica; Inibidores da angiogênese; Nanotecnologia; Nanopartículas; Injeções intravítreas; Coelhos
INTRODUCTION
Retinal diseases, such as age-related macular degeneration (AMD), diabetic retinopathy, and retinal vein occlusions present with the proliferation of exudative processes and abnormal retinal vessels, and they are responsible for the majority of visual impairment cases in developed countries(1-3).
The advent of antiangiogenic drugs signaled a breakthrough in the treatment of these diseases with favorable functional and anatomical outcomes and increased visual loss prevention rates(4-8).
An optimal effective treatment for retinal neovascular diseases with fewer injections and higher angiogenic inhibition has not yet been established. Several studies are being conducted to discover drugs that can inhibit the largest number of angiogenesis mediators, and that can remain available in the vitreous for prolonged periods.
The use of nanotechnology in the medical field has begun a new era of emerging therapeutic advances. In ophthalmology, promising research has focused on the use of nanoparticles for improving drug delivery to specific sites(9).
The advantages of nanoparticles include their increased apparent solubility and the sustained release of the drug. This unique property is extremely useful in treating chronic conditions such as AMD, diabetic retinopathy, and retinal vein occlusions.
On the basis of the possibility of development of a sustained delivery system for treating retinal diseases, we aimed to evaluate the retinal toxic effects of sunitinib (at two different concentrations) and different nanoparticle preparations in an animal model. We chose sunitinib because of its marked antiangiogenic activity and suitable physicochemical molecular properties for nanoparticle preparation. We tested solid lipid nanoparticles (SLNs) and polymeric nanocapsules, which can both efficiently incorporate the sunitinib molecule.
We performed retinal analyses for toxic effects using retinal electrophysiology and light microscopy histopathology of the albino rabbit retinas.
METHODS
Study design
We conducted a controlled experimental study using 20 albino rabbits. The rabbits were separated into four groups of five each. We applied an intravitreal injection to one eye in each rabbit, and the other eye was used as a control. Each group received a different preparation as follows: sunitinib 0.1 ml (1 mg/ml), sunitinib 0.1 ml (10 mg/ml), a dispersion containing SLN 0.1 ml, or a dispersion containing NC 0.1 ml. We evaluated retinal toxic effects 26 days after the injections.
Animals
We treated all animals used in the experiments in accordance with the regulations of the Association for Research in Vision and Ophthalmology.
Our sample consisted of 20 albino rabbits, which were distributed by a random drawing into the following four groups: group 1 rabbits were injected with intravitreal sunitinib 0.1 ml (1 mg/ml), group 2 with intravitreal sunitinib 0.1 mL (10 mg/ml), group 3 with 0.1 ml of a drug-free dispersion containing SLNs, and group 4 with 0.1 ml of a drug-free intravitreal dispersion containing polymeric nanocapsules.
We performed indirect binocular ophthalmoscopy (BIO) with a 20-diopter lens for evaluating the vitreous humor, retina, optic nerve, and possible transparency changes on the day before the injections and 26 days after the procedures. We submitted animals without changes after the injections for electroretinographic studies.
Euthanasia of animals
For euthanasia (26 days after the injections), the animals were sedated to a moderate level using xylazine hydrochloride 2%, and a sodium thiopental solution 50 mg/kg diluted to 2.5% was intravenously used.
Under deep anesthesia, an injection of potassium chloride 10% was administered. After confirming their death, the rabbit bodies underwent enucleation and eye removal.
Electroretinography
We used a Roland Consult Ganzfeld Q450 (Mainz, Germany) electroretinograph. All rabbits in the study underwent total field electroretinography (ERG) after pupillary dilation, before intravitreal injection of each substance, and at the end of the study (26 days later) prior to euthanasia. Our protocol followed the guidelines of the International Society for Clinical Electrophysiology of Vision.
The impedance was maintained equal to or lower than 5 kOhm. The photopic stage to assess the cone system was held in the dark after adaptation to light for 10 minutes (with light behind the dome).
All stimulations were repeated three times with photopic flashes at frequencies of 0.5, 1.0, 2, 3, 5, 10, 15, and 30 Hz, ranging in intensity from -2.50 to + 0.5 cd/m2. The biological signal was amplified and filtered with band-pass, high-pass, and low-pass. The analysis time was 20 ms/div for 1.0 Hz and 50 ms/div for frequencies of 5, 10, 15, and 30 Hz.
Amplitudes (µw) peak-to-peak wave and time latency (ms) were analyzed. The responses were analyzed at 0.5, 1.0, 2, and 3 Hz, in amplitude of 100 microvolts/div and latency of 50 ms/div, and frequencies at 5, 10, 15, and 30 Hz, in amplitude of 100 micro volt/div and 20 ms latency/div.
Slide preparation
The slides for the histopathological analysis were stained using hematoxylin and eosin HE, and images were captured under a 20× magnifying lens (0.40” focus range) in a Nikon Eclipse 50i (Nikon Instruments, USA) microscope with the Motic Images Plus image-capture system.
Sunitinib preparation
The fractionation of sunitinib maleate salts was carried out in the vertical laminar flow chamber under sterile conditions. The sterile solution was prepared in a balanced saline solution.
Solid lipid nanoparticle preparation
We produced the SLNs using a micro emulsion dilution technique. Initially, we added 250 µL of ultrapure water to a mixture of molten lipid (stearic acid) and surfactants (sodium taurodeoxycholate and phosphatidylcholine). The mixture was heated under a magnetic stirrer until formation of a micro emulsion. Next, we added the micro emulsion, drop by drop into a flask containing 19 ml of TES buffer at pH 7.4, and cooled the mixture while stirring in an ice bath (2°C-4°C) in an Ultra-turrax®, German, mixer at 13.400 rpm for 10 minutes. SLN formation occurs immediately upon addition of the micro emulsion to the cold buffer by lipid solidification. We assessed the size and polydispersity index (PDI) of the nanosystems in a Zetasizer Nano-S equipment using a dynamic light scattering technique. The particles were sterilized by sterile filtration through 0.22-µm filters (Millex®, Millipore, USA).
Polymeric nanocapsule preparation
We prepared the polymeric nanocapsules using the interfacial deposition of preformed polymer method. The organic phase comprised of Miglyol® (Capric and caprylic acid triglycerides), PLGA 50:50 (polymer), and soy phosphatidylcholine (surfactant) dissolved in acetone. The aqueous phase comprised of Poloxamer F-127 and F68 (surfactant) dissolved in pH 7.4 phosphate buffer. We poured the organic phase over the aqueous phase and maintained the mixture under a magnetic stirrer for 30 minutes. Subsequently, the organic solvent was removed from the formulation in a rotary evaporator and the solution was concentrated to a final volume of 10 ml by water removal. The formation of the nanocapsules occurred upon removal of the organic solvent and interfacial deposition of polymer. The particles were sterilized by sterile filtration through a 0.22-µm filter (Millex®).
The nanocapsules had a mean diameter of 130 nm and the NLS a mean diameter of 140 nm. The two nanosystems were monodisperse (PDI 0.08 for nanocapsules, PDI 0.20 for SLN). Both formulations exhibited physiological pHs (pH 7.4).
Analysis of results and statistics
For statistical analysis, we tabulated the data into a Microsoft® Office Excel® 2007 spreadsheet. The spreadsheet was imported for analysis using the Stata/SE 11.1 software for Windows.
We used the paired t test in the majority of cases, and the Wilcoxon signed-rank test when the variance difference between samples was large. A p value of 0.05 was considered to indicate a statistically significant change in the retina after the intravitreous injection.
RESULTS
The sample consisted of 20 New Zealand albino male rabbits, the rabbits received injections in their right eyes.
All animals underwent ophthalmologic evaluations for verification of clinical alterations and retinal toxic effects at baseline and 26 days after the intravitreal injections.
Clinical ophthalmic examination 26 days after intravitreal injection in the rabbits’ eyes
In Groups 1 and 2 (sunitinib 1 and 10 mg/ml, respectively) all eyes showed some amount of drug deposition in the posterior lens capsule, was observed 26 days after the injection. Two eyes in group 2 developed subcapsular cataracts, 26 days after the injection.
In Group 3, 2 eyes had posterior subcapsular cataracts and two eyes showed discrete deposition of particles in the posterior lens capsule.
In Group 4, all eyes presented posterior lens clouding and substance deposition in the vitreous.
Statistical evaluation of electrophysiology in the rabbits’ retina
The responses were verified at the initial examination and 26 days after the injections in all groups. We found significant morphological changes and wave amplitude differences in the ERG a and b wave evaluations. The electroretinograms revealed the presence of waves detectable throughout the course of the examination in Groups 1, 2, and 3, with normal responses, in terms of amplitude and also in the implied time of waves a and b.
We noted no significant reduction or absence of ERG response dependent on the stimulus used. The measurement of a and b waves revealed no morphological or functional alterations in Groups 1, 2 or 3 (p=0.428) (Figure 1).
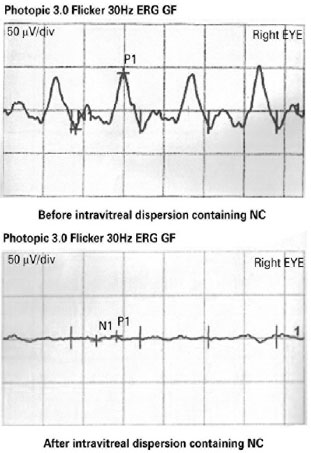
On the contrary, we found statistically significant ERG wave changes in morphology and in the amplitude and implicit times when comparing pre- and post-intravitreous injection exams of each treated eye (p=0.004).
Histopathological evaluation of the rabbits’ retina
We found that the eyes that underwent intravitreal injections of sunitinib and SLN presented retinas with a well preserved general histo-architecture without inflammatory cells detected in the subretinal space (Figure 2).
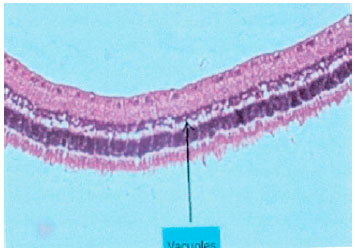
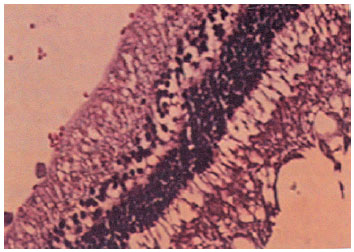
In contrast, the eyes that underwent intravitreal NC injections presented retinas with preserved general histo-architecture, but the inner retinal layers presented degenerative changes: swollen cell nuclei with marked edema and the formation of cytoplasmic vacuoles. Again, no inflammatory cells were detected in the subretinal space (Figure 3).
DISCUSSION
The discovery of ocular angiogenesis factors enabled the development of different drugs that inhibit the process. Proliferative retinopathies are characterized by increased vascular permeability and proliferation of new blood vessels. Two key agents involved in these mechanisms are VEGF and PDGF(10-16).
Sunitinib is a tyrosine kinase inhibitor that results in decreased expression of both VEGF and PDGF. This property may be valuable for the treatment of neovascular eye diseases, either in monotherapy or in combination with other drugs(17).
Research on the ophthalmological uses of sunitinib have shown that it does indeed inhibit neovascularization(17-19).
However, to our knowledge, the intravitreal use of sunitinib in the treatment of neovascularization diseases in humans has not yet been reported. We chose to begin by assessing the safety of intravitreal sunitinib use in an animal model. The advantage of sunitinib use over classic antibodies used for VEGF inhibition is that sunitinib is a cyclic GMP inhibitor that when added to nanoparticles may retain its pharmacological properties for prolonged periods of time.
In the present study, we found no significant electroretinogram changes after sunitinib injections. However, the histopathological findings did reveal the presence of discrete vacuoles, which may be attributed to artifacts from the sample preparation. Two eyes from these rabbits showed slight ERG changes, but we disregarded those because the changes correlated with lens opacities of the lens.
Similar to our results, cataracts have been seen after the intravitreal injection of celecoxib in rabbit eyes, but the researchers concluded that this toxic effect is less likely in human eyes, which have a larger amount of vitreous humor and present more syneresis that would facilitate the deposition of the drug in a position farther from the lens(20).
Also, another reason for the development of cataracts in the eyes subjected to sunitinib injection is that this drug is a salt and its ionicity may have caused a functional Na+/K+ATPase pump change. This process also occurs when using corticosteroids, topical or systemic, that affect the transport of water and increase the cationic permeability of the lens, causing hydration and decreased transparency(21).
Our retinal electroretinographic results were similar to those published by Dib et al in terms of animal model study characteristics(22).
Sunitinib can be dissolved in colloidal systems, it can be trapped or encapsulated in a matrix, and it can be present in different compositions such as in nanoparticles, nanocapsules, or nanospheres(23).
Therapy with nanotechnology has major advantages when compared with conventional drug treatments. Their sustained-release characteristics and targeted delivery make nanotechnological substances promising for vasoproliferative ocular diseases(23,24).
The inner and outer blood-retinal barriers block the absorption of drugs to the posterior segment of the eye. Consequently, the intraocular concentrations of conventional drugs fail to provide full efficacy. New forms of drug delivery to the posterior eye segment are being studied to provide better drug availability to the intraocular tissues. Diseases such as AMD, diabetic retinopathy, and retinal vein occlusions may benefit from the use of nanotechnology with its directed and sustained-release characteristics(25).
During the past decade, nanoparticle studies have shown promising results when evaluating the delivery characteristic of drugs to the target tissues. Bourges et al. showed that intravitreal-injected nanocapsules of different sizes accumulate in the retinal pigmented epithelium (RPE) and can be detected there for up to 4 months after administration in rabbit eyes. The PLA and PLGA polymers in the particle possibly modified the structure of the vitreoretinal interface leading to a break in the internal limiting membrane (ILM) and allowing particle penetration between the retinal layers to reach the RPE. The other possibility is that inflammation induced by the procedure modified the permeability and the absorption mechanism through the ILM. On the basis of these results, nanodrugs for treating posterior segment conditions affecting the RPE may be especially promising(26).
We designed this study for evaluating the toxic retinal effects of polymeric nanocapsules and SLNs that can offer sustained drug release in the posterior eye segment. The nanostructure formulations can be formed using various substances and can be prepared applying different techniques. Nanocapsules are reservoir-type systems in which it is possible to identify a core/differential matrix that may be solid or liquid. On the contrary, SLNs are a monolithic colloidal carrier system designed to encapsulate, protect, and deliver lipophilic functional components, such as drugs and bioactive lipids(27).
The results obtained in this study demonstrated that all the rabbit eyes that received intravitreal nanocapsule injections developed posterior lens opacification and substance deposits into the vitreous body. Also, the ERG and histopathological analysis of those retinas showed morphological changes and wave amplitude differences in the a and b wave evaluations. In contrast, the eyes injected with SLN injections had no ERG or histopathological changes. On the basis of these results, the substances used in the preparation of the nanocapsules or pH changes induced by degradation of the polymer may have led to the changes found in the retinas. The SLNs, on the contrary, are made up of nontoxic components and do not require the use of organic solvents in their preparation. Another advantage of SLNs is that their lipid matrix may be produced from biodegradable lipids, which are also nontoxic(28,29).
Our findings open the door to the possibility of using sunitinib (a drug that can inhibit two important angiogenesis pathways) in a sustained delivery system that can probably also maintain its pharmacological properties as a therapeutic approach for treating vasoproliferative ocular diseases; further research is warranted.
REFERENCES
1. Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987; 235(4787):442-7.
2. Bosco A, Lelário AC, Soriano D, Santos RF dos, Massote P, Galvão D, et al. Retinopatia diabética. Arq Bras Endocrinol Metab. 2005; 49(2):217-27.
3. Valiatti FB, Crispim D, Benfica C, Valiatti BB, Kramer CK, Canani LH. Papel do fator de crescimento vascular endotelial na angiogênese e na retinopatia diabética. Arq Bras Endocrinol Metab. 2011; 55(2):106-13.
4. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14): 1419-31.
5. Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group. Ranibizumab for predominantly classic neovascular age-related macular degeneration: Subgroup Analysis of First-year ANCHOR Results. N Engl J Med. 2006;355(14):1432-44.
6. Bashshur ZF, Haddad ZA, Schakal A, Jaafar RF, Saab M, Noureddin BN. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol. 2008;145(2):249-56.
7. Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci. 2007;104(47):18363-70.
8. Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171-85.
9. Diebold Y, Calonge M. Applications of nanoparticles in ophthalmology. Prog Retinal Eye Res. 2010;29(6):596-609.
10. Adamis AP, Miller JW, Bernal MT, D’amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445-50.
11. Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480-7.
12. Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, De Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81(2):154-62.
13. Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997; 277(5323): 242-5.
14. Furuhashi M, Sjöblom T, Abramsson A, Ellingsen J, Micke P, Li H, et al. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004;64(8): 2725-33.
15. Damico FM. Angiogênese e doenças da retina. Arq Bras Oftalmol. 2007;70(3):547-53.
16. Lima Filho AA, Dantas AM, Sallum JM, Ferreira Filho NF, Marback RL. Bases da oftalmologia. Rio de Janeiro: Cultura Médica; Guanabara Koogan, 2008. v. 1. [Série Oftalmologia Brasileira, Conselho Brasileiro de Oftalmologia].
17. Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme M, Javaloy J, Alió JL. Inhibition of corneal neovascularization by topical bevacizumab (Anti-VEGF) and Sunitinib (Anti-VEGF and Anti-PDGF) in an animal model. Am J Ophthalmol. 2010;150(4):519-28.
18. Roskoski Jr. R. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007; 356(2):323-8.
19. Ko BY, Kim YS, Baek SG, Lee GW, Kim JM, Jean WS, et al. Inhibition of corneal neovascularization by subconjunctival and topical bevacizumab and sunitinib in a rabbit model. Cornea. 2013;32(5): 689-95.
20. Kim SJ, Toma H, Shah R, Kompella UB, Vooturi SK, Sheng J. The safety, pharmacokinetics, and efficacy of intraocular celecoxib. Invest Ophthalmol Vis Sci. 2014;55(3):1409-18.
21. Bucala R, Fishman J, Cerami A. Formation of covalent adducts bethewen cortisol and 16 alpha-hydroxyestrone and protein: possible hole in the pathogenesis of cortisol toxicity and systemic lupus erythematosus. Proc Natl Acad Sci. 1982;79:3320-4.
22. Dib E, Maia M, Lima AS, Costa EP, Moraes-Filho MN, Rodrigues EB, et al. In vivo, in vitro toxicity and in vitro angiogenic inhibition of sunitinib malate. Cur Eye Res. 2012;37(7):567-74.
23. Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13(3/4):144-51.
24. Vanderwoot J, Ludwig A. Ocular drug delivery: nanomedicines application. Nanomedicine. 2007;2:11-21.
25. Xu Q, Kambhampati SP, Kannan RM. Nanotechnology approaches for ocular drug delivery. Middle East Afr J Ophthalmol. 2013;20(1): 26-37.
26. Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44(8):3562-9.
27. Helgason T, Awad TS, Kristbergsson K, McClements DJ, Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J Colloid Interface Sci. 2009;334(1):75-81.
28. Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLNTM) dispersions. Int J Pharm. 1998;168(2):221-9.
29. Parhi R, Suresh P. Production of solid lipid nanoparticles-drug loading and release mechanism. J Chem Pharm Res. 2010;2(1):211-27.
Submitted for publication:
October 5, 2017.
Accepted for publication:
March 13, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose
Approved by the following research ethics committee: Universidade Federal de Goiás (# 062/2011)