

Grazielly Martins Peixoto de Oliveira1; Walton Nosé2; Maria Lúcia Zaidan Dagli3; Vanessa Vieira Cordeiro1; Nicolas Cesário Pereira1; Adriana S. Forseto1,2
DOI: 10.5935/0004-2749.20180077
ABSTRACT
Purpose: The aim of this study was to compare the corneal cell viability and thickness of LASIK flaps created by 3 femtosecond lasers in eye-bank human corneas. Methods: Forty-five eye-bank human sclerocorneal buttons (15 corneas in each group) were examined after the creation of 120 µm-thick laser-assisted keratomileusis (LASIK) flaps with 150kHz iFS IntraLaseTM (IL), Z6 Femto LDVTM (LDV), or 200kHz WavelightTM FS200 (FS200). The thickness of the flaps was measured using anterior segment optical coherence tomography (AS-OCT; VisanteTM). Cell viability was blindly evaluated with immunohistochemistry for keratocyte apoptosis using anti-caspase 3 antibodies. Results: The standard deviation from the intended flap thickness was less than 10 µm in all the groups. There was a statistically significant difference in corneas treated with LDV and IL with regard to the flap thickness horizontally at +3.00 mm (p=0.0124), -0.5 mm (p=0.0082), and -1.00 mm (p=0.0425) from the corneal vertex and +0.5 mm from the flap edge (p=0.0240), and those treated with LDV and FS200 with regard to the flap thickness horizontally at -0.5 mm from the corneal vertex (p=0.0082). The mean keratocyte apoptosis numbers were 13.09 ± 1.10, 15.59 ± 3.28, and 17.72 ± 1.49 in corneas treated with IL, FS200, and LDV, respectively (p<0.001). Conclusion: All 3 assessed femtosecond lasers provided predictable LASIK flap thickness. The mean stromal keratocyte apoptosis number was low in all groups.
Keywords: Keratomileusis, laser in situ; Femtosecond; Tomography, optical coherence, Anti-caspase 3; Cornea; Eye-bank
RESUMO
Objetivos: Comparar a viabilidade celular e a espessura do disco de LASIK confeccionado por três laseres de femtosegundo, em córneas humanas de banco de olhos. Métodos: Quarenta e cinco botões córneo-esclerais humanos de banco de olhos (15 córneas em cada grupo) foram examinados, após a criação de disco de LASIK com 120 µm de espessura, utilizando-se o iFS IntraLase® 150kHz (IL), o Femto LDV® Z6 (LDV), ou o Wavelight® FS200 200kHz (FS200). Tomografia de coerência óptica do seguimento anterior (OCT Visante®) foi usada para medir a espessura dos discos. A viabilidade celular foi avaliada por meio de imuno-histoquímica para apoptose dos ceratócitos, com anti-caspase 3. Resultados: O desvio padrão da espessura planejada do disco foi inferior a 10 µm em todos os grupos. Houve diferença estatisticamente significante da espessura do disco horizontalmente a +3,00 mm (p=0,0124), -0,5 mm (p=0,0082) e -1,00 mm (p=0,0425), a partir do vértice corneal, e a +0,5 mm (p=0,0240), a partir da borda do disco, em córneas tratadas por LDV e IL, e horizontalmente a -0,5 mm a partir do vértice corneal, entre LDV e FS200 (p=0,0082). A média de apoptose dos ceratócitos foi (13,09 ± 1,10), (15,59 ± 3,28) e (17,72 ± 1,49), em córneas tratadas pelo IL, FS200 e LDV, respectivamente (p<0,001). Conclusão: Todos os três laseres de femtosegundo estudados produziram disco de LASIK com predictibilidade de espessura. A média de apoptose dos ceratócitos foi baixa em todos os grupos.
Descritores: Ceratomileuse assistida por excimer laser in situ; Femtosegundo; Tomografia de coerência óptica; Anti-caspase 3; Córnea; Banco de olhos
INTRODUCTION
The predictability and accuracy of laser-assisted keratomileusis (LASIK) flap thickness, diameter, and morphology, as well as the homogeneity of its interface can alter refractive outcomes and the risks of complications from the procedure(1-3). LASIK flap thickness can be measured with anterior segment optical coherence tomography (AS-OCT), and this approach is reliable and reproducible(1). Zone cell damage in the LASIK retroablation area can also alter refractive outcomes, as it interferes with the corneal wound healing process and with remodeling(4). Apoptosis of stromal keratocytes is an early corneal wound-healing response after injury of the cornea. It can be used to analyze cell damage and viability, and mean keratocyte apoptosis is considered to be proportional to corneal remodeling(4,5). Caspase 3 plays a central role in the proteolytic cascade of apoptosis, and it is considered as a reliable marker to identify apoptotic cells(6).
The LASIK flap is created with a mechanical microkeratome or with a femtosecond (FS) laser(7). While many studies have compared a microkeratome flap with a laser flap, there have been limited comparisons among flaps obtained with FS systems(8,9).
The aim of this study was to compare the corneal cell viability and thickness of LASIK flaps created by 3 FS lasers with eye-bank human corneas.
METHODS
This study was approved by the Ethics Committee of Sorocaba Eye Bank, Sorocaba Ophthalmologic Hospital. Forty-five eye-bank human sclerocorneal buttons from Sorocaba Eye Bank (Sorocaba, SP, Brazil), which were preserved in Optisol-GSTM (Bausch and Lomb Surgical, Rochester, NY, USA) at between 2 and 8°C up to 7 days after enucleation and were not considered for transplantation because of a positive serology status, were selected. The buttons were evaluated using the slit lamp Topcon SL-D7 (Topcon Medical Systems, Inc., Oakland, NJ, USA) before and after preservation, and the number and morphology of corneal endothelial cells were analyzed using the Konan Eye Bank Kerato Analyzer (Konan Medical, Inc., Nishinomiya, Japan). The intensity of corneal stromal edema was classified as 0, +1, +2, +3, or +4, where 0 corresponded to the absence of edema and +4 corresponded to the maximum intensity of edema. Corneas with stromal edema intensity of 0 or +1 and with at least 2,000 endothelial cells in a regular mosaic format were selected. Corneas were divided into the following 3 groups (15 corneas in each group) according to the type of FS laser platform used to manufacture the LASIK flaps: 150kHz iFS IntraLaseTM (IL) (Abbott Medical Optics, Santa Ana, CA, USA); Z6 Femto LDVTM (LDV) (Ziemer Ophthalmic Systems AG, Port, Switzerland); and 200 kHz WavelightTM FS200 (FS200) (Alcon Laboratories, Inc., Fort Worth, TX, USA) groups.
Flap creation
Each cornea was removed from Optisol-GSTM and fixed in a disposable artificial anterior chamber (AAC) (Katena Products, Inc., Denville, NJ, USA for the IL and FS200 groups, and Ziemer Ophthalmic Systems AG for the LDV group), which was filled with balanced salt solution (BSS, Alcon Laboratories, Inc.) with a pressure of approximately 30 mmHg(10). Epithelial debridement of the cornea was performed with a disposable crescent blade (Katena Products, Inc.).
All FS laser platforms were programmed to create a flap having a thickness of 120 µm and a diameter of 9.00 mm.
Each sclerocorneal button was applanated using the disposable interface contact lens of the system without the need for a suction ring. An IntershieldTM spacer (Ziemer Ophthalmic Systems AG), which is a sheet of plastic positioned between the laser handpiece and the cornea, was used in the LDV group(11).
The FS laser settings for creating the LASIK flaps in all groups are described in table 1(12,13).
Flap evaluation
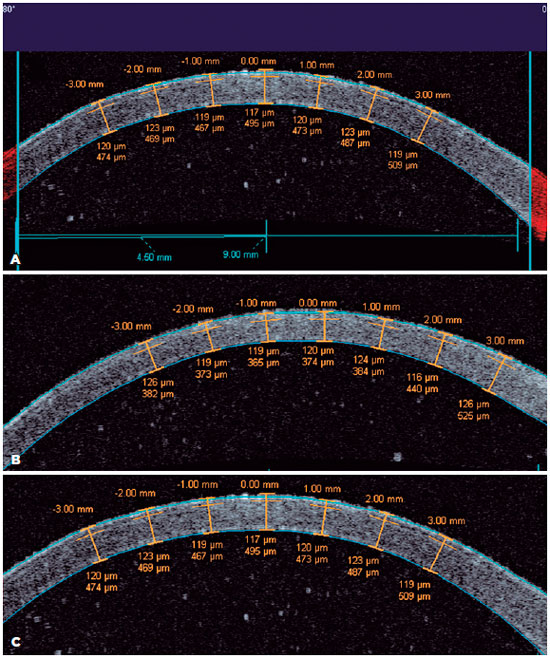
Two cross-sectional axial scans (10.0 mm in size) were performed horizontally and vertically with the high-speed AS-OCT system VisanteTM (Carl Zeiss Meditec, Dublin, CA, USA). The axial scan frequency was 2,000 Hz, and each image was composed of 256 A-scans (axial and transversal resolutions of 18.0 µm and 60.0 µm, respectively)(1). LASIK flap thickness was measured in the enhanced high-resolution cornea mode at the center and at 10 points (Figure 1) in each horizontal and vertical image (distances of 0.5 mm, 1.0 mm, 2.0 mm, and 3.0 mm from the corneal vertex, and 0.5 mm from the flap edge). With the flap hinge as a reference at 90° (superior), data were collected horizontally at 0° (+0.5 mm, +1.0 mm, +2.0 mm, and +3.0 mm from the corneal vertex, and +0.5 mm from the flap edge) and 180° (-0.5 mm, -1.0 mm, -2.0 mm, and -3.0 mm from the corneal vertex, and -0.5 mm from the flap edge), and vertically at 90° (+0.5 mm, +1.0 mm, +2.0 mm, and +3.0 mm from the corneal vertex, and +0.5 mm from the flap edge) and 270° (-0.5 mm, -1.0 mm, -2.0 mm, and -3.0 mm from the corneal vertex, and -0.5 mm from the flap edge)(1,14,15). Immediately after the imaging study, the flap was lifted with a LASIK spatula (Katena Products, Inc.), and it was repositioned for immunohistochemical examination. Corneas were kept refrigerated in Optisol-GSTM medium for 12 h after LASIK flap creation until fixation. After 12 h of fixation in 10% formaldehyde, the specimens were cut and embedded into paraffin blocks, and 5 µm sections were created from the paraffin blocks(16) and were mounted on silane-coated slides, dried, deparaffinized in xylene, and rehydrated in ethanol. Sections then underwent heat-induced antigen retrieval with a microwave oven (3 x 5 min cycles in 10 mM Tris-EDTA buffer [pH 9] at 650 W). Endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 min. Sections were carefully wiped to absorb excess moisture. Using a pen draw (Dakocytomatic, Dako, CA, USA) on the glass slide, a complete circle was made around each section. This hydrophobic barrier confined reagents to the section, thus preventing them from spreading. Briefly, the sections were rinsed with TBS (0.01 M Tris, 0.15 M NaCl, pH 7.6) in a solvent tank. Slides were incubated with the primary antibody for caspase 3 (1:100; Uniscience, Fort Garry Ind. Park, Winnipeg, Canada). The primary antibody solution or negative control completely covered the sections. The slides were placed in a humidified container and were incubated at 4°C overnight (around 16 h)(6). The slides were rinsed in PBS thrice (each rinse was for 5 min). Sections were incubated with biotinylated goat anti-rabbit IgG (1:400 in PBS; secondary antibody; Vector Laboratories Inc., Burlingame, CA, USA) for 30 min at room temperature. The slides were rinsed in PBS thrice (each rinse was for 5 min). Sections were incubated in FITC-streptavidin for 30 min, with the slides protected from light. The slides were rinsed in PBS thrice (each rinse was for 5 min), and a cover slip was placed with aqueous mounting medium and was sealed with nail polish. Slides were scanned using the fluorescence microscope Nikon Eclipse® E-800 (Nikon, Melville, NY, USA) under 400× magnification, and 5 fields were imaged and stored by a computerized image analysis program (Image Pro Plus, Nikon, Melville, NY, USA) prior to cell counting. The locations of the 5 fields were as follows: the central LASIK retroablation zone; 2 fields distance from the central field to the right; 2 fields distance from the central field to the left; immediately right of the flap edge; and immediately left of the flap edge. A blinded examiner counted the keratocyte apoptotic cells.
Statistical analysis
The Kruskal-Wallis test with Dunn’s post-hoc test was used to evaluate statistical differences in the thickness of the flaps and keratocyte apoptosis. All statistical analyses were performed using Prism 5 software (GraphPad Software Inc., San Diego, CA, USA), and a p-value <0.05 was considered significant.
RESULTS
Fifteen eye-bank corneas were allocated in each of the 3 groups (LDV, FS200, and IL groups). There were no differences with regard to preservation time and endothelial cell count among the groups. Additionally, no intraoperative flap complications were observed in any of the groups.
The mean flap thickness measurements at all points in the groups are shown in tables 1 and 2, and figures 2 and 3. There were statistically significant differences in the corneas between the LDV and IL groups with regard to the flap thickness horizontally at +3.00 mm (p=0.0124), -0.5 mm (p=0.0082), and -1.00 mm (p=0.0425) from the corneal vertex and at +0.5 mm from the flap edge (p=0.0240) (Table 1). Additionally, there were statistically significant differences in the corneas between the LDV and FS200 groups with regard to the flap thickness horizontally at -0.5 mm from the corneal vertex (p=0.0082) (Table 2). No statistically significant difference was found vertically (Table 3).
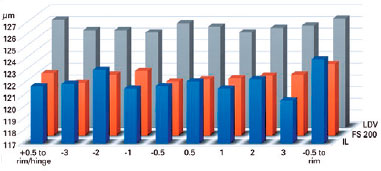
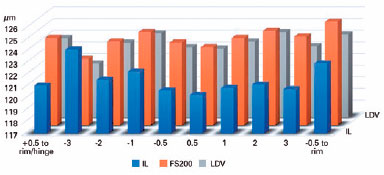
The mean keratocyte apoptosis numbers were 13.09 ± 1.10, 15.59 ± 3.28, and 17.72 ± 1.49 in corneas treated with IL, FS200, and LDV, respectively (p<0.001), with significant differences between LDV and IL, and between LDV and FS200 (Table 4).
DISCUSSION
Flap thickness regularity and predictability are important for optimal LASIK surgery outcomes. A thin corneal flap maintains the integrity of the epithelium and Bowman’s layer and a thick residual stroma, reducing complications, such as corneal ectasia(2).
FS laser technology has the advantage of producing a LASIK flap with predictability, a more uniform thickness throughout(3), and a more planar morphology(3,14). The current available FS laser systems can be classified into the following 2 categories: high energy/low repetition rate (FS200 and IL used in this study) and low energy/high repetition rate (LDV used in this study). The accuracy of the flap cut may be altered by the formation of a stromal opaque bubble layer (OBL), which is more likely with high-energy devices(9,10,17). In this study, no clinical difference in OBL formation was observed among the groups. Comparisons between 15- and 30kHz FS IntraLaseTM devices in eye-bank corneas with regard to the creation of partial thickness donor corneal buttons for lamellar keratoplasty showed a more homogeneous interface with the lower energy per pulse device (FS 30 kHz)(18).
The standard deviation (SD) of corneal flap thickness may range from 22 to 30 µm with a microkeratome(7,15,19,20) and from 4 to 18.4 µm with FS(3,21). In this study, the SD of corneal flap thickness ranged from 4.73 to 6.74 µm with LDV, from 3.36 to 8.14 µm with FS200, and from 2.93 to 7.32 µm with IL.
All of the FS laser platforms created planar LASIK flaps, with no thickness variation from the center to the periphery and excellent thickness predictability. LDV was slightly less predictable, but this was only at 4 of the 20 specific points measured (+3.00 mm, -0.50 mm, and -1.00 mm from the corneal vertex and +0.50 mm from the flap edge horizontally). This may be explained by the overlapping of laser spots during cutting with LDV. Another possible reason for this finding is the different AACs used to create the LASIK flaps with LDV. The flexible LDV laser arm and handpiece could also play roles in this finding, but only during surgery, as a uniform pressure against the eye is required during cutting. Pressure variation could reduce precision. In this experimental study, however, the laser handpiece was laid on the AAC with no external pressure. Therefore, it appears more reasonable that the time-domain OCT used in this study was not able to accurately measure the flap thickness at all extensions considering its depth of field of 18 µm.
A prospective comparative case series using Fourier-domain OCT, which has a depth resolution of 5 µm, showed a uniform LASIK flap with FS 60kHz IntraLaseTM and with 200kHz VisuMaxTM FS laser (Carl Zeiss Meditec) and a meniscus-shaped flap with Femto LDVTM Crystal Line, as well as low thickness variation between the periphery and center with IntraLaseTM(14). Although we found few flap thickness differences, the results of this study demonstrate a significant improvement with LDV when compared to previous equipment, as the device creates a far more uniform flap, without a meniscus shape.
The cellular effects of the laser are also important for LASIK surgery outcomes, as they interfere with the corneal wound healing process and remodeling(4). Numerous studies have used immunochemistry to detect apoptosis markers (Fas, Fas ligand, caspase 3, Bcl-2, and Bax)(4-6,16). Caspase 3 plays a central role in the proteolytic cascade in apoptosis, and it is considered an early and specific marker to detect cells destined to die by apoptosis, as it can detect these cells before the appearance of morphological alterations (fragmentation of the nucleus, formation of apoptotic bodies, and chromatin condensation)(6). The expression of apoptosis has been shown to be associated with keratocyte depletion and regeneration, corneal endothelial recovery, and wound healing and remodeling(5). Corneal wound healing after LASIK has an important role in the development of surgery complications, such as overcorrection and undercorrection(22). Thus, it is expected that greater keratocyte apoptosis is associated with greater corneal remodeling and lower safety and effectiveness of the refractive procedure. Apoptosis and inflammatory cell infiltration are proportional to the amount of energy with the FS system(23,24). Four hours after corneal epithelial injury, it is possible to observe the peak of stromal keratocyte apoptosis(23,25) and stromal cell inflammatory infiltration, and these remain high even at 24 h after the injury(23,24,26).
In our study, there was a slight difference in keratocyte apoptosis among the groups. At 12 h after treatment, there was no evidence of inflammatory cell infiltration, probably because corneas without blood supply were used.
Higher energy per pulse and the total energy released in the earlier high-energy FS systems may cause more stromal keratocyte death than that with low-energy FS systems, especially through necrosis and inflammatory cell infiltration(8,23,26,27). Necrosis was greater in rabbit corneas treated with FS 15kHz IntraLaseTM than with FS 30- and 60 kHz IntraLaseTM for LASIK flap creation(26). Rabbit corneal keratocyte apoptosis marked with the terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, which was evaluated after LASIK flap creation with FS 60kHz IntraLaseTM, showed mean apoptotic cell numbers of 31.9 ± 7.1, 22.2 ± 1.9, and 17.9 ± 4.0 with high, intermediate, and low energy, respectively(24). Current FS systems with high repetition rates can reduce the energy required, with a consequent decrease in bubble layer formation, decrease in inflammation, decrease in the time to flap creation, and increase in the ease of lifting the flap(17,28). The production of LASIK flaps with FS or microkeratome has been shown to cause similar corneal stromal cellular effects with current devices(8,27-30). Corroborating the findings of previous research, the mean keratocyte apoptosis number in our study was low in all groups. This is partly associated with significant improvements in the FS laser systems used in this study. Additionally, the finding is associated with the use of caspase 3 for assessment, as caspase 3 is a more specific marker to detect apoptosis than other approaches used in previous studies (TUNEL). Thus, caspase 3 is expected to mark a lower number of cells when compared to TUNEL, as TUNEL marks not only apoptotic cells but also necrotic cells(31).
In our study, the mean keratocyte apoptosis number was the highest in corneas treated with LDV (17.72 ± 1.49), followed by those treated with FS200 (15.59 ± 3.28) and those treated with IL (13.09 ± 1.10). Although low energy is provided by LDV, it is possible that overlapping of its laser spots because of its high repetition rate may play a role in this apoptotic reaction. On the other hand, although both the FS200 and IL systems are classified as high-energy and low-repetition FS systems, both are far beyond the earlier studied FS models of 15 kHz. A lower apoptosis rate is associated with less corneal remodeling, which increases the chance of achieving the refractive goals of treatment (with less overcorrection, undercorrection, and surgery complications), as it depends on corneal healing stability(4,22).
It is important to consider the limitations of assessing cellular responses with human sclerocorneal buttons from an eye bank. In addition to animal models, interesting experimental human organ models are being developed. Although there is currently no human corneal model with a stable 3D layer conformation, to allow studies on LASIK flaps, the most promising organ systems being developed are human iPS cell-derived organoids involving sequential rounds of differentiation programs(32). These organoids share features of the developing cornea, harboring 3 distinct cell types with expressions of key epithelial, stromal, and endothelial cell markers. However, currently, they do not have the same biomechanics as those of a real human cornea, and they are not considered a good model for refractive surgery research yet. In the future, a better human experimental model may be developed to study the inflammatory and cellular responses of corneal healing after surgery.
Although previous studies correlated the cellular effects of laser with LASIK surgery outcomes(4), this is an experimental study and does not have clinical validity.
In conclusion, all 3 studied FS lasers provided predictable LASIK flap thickness and a low mean keratocyte apoptosis number.
REFERENCES
1. von Jagow B, Kohnen T. Corneal architecture of femtosecond laser and microkeratome flaps imaged by anterior segment optical coherence tomography. J Cataract Refract Surg. 2009;35(1):35-41.
2. Chen HJ, Xia YJ, Zhong YY, Song XL, Chen YG. Anterior segment optical coherence tomography measurement of flap thickness after myopic LASIK using the Moria one use-plus microkeratome. J Refract Surg. 2010;26(6):403-10.
3. Cummings AB, Cummings BK, Kelly GE. Predictability of corneal flap thickness in laser in situ keratomileusis using a 200 kHz femtosecond laser. J Cataract Refract Surg. 2013;39(3):378-85.
4. Li Q, Ashraf MF, Bekoe NA, Stark WJ, Chan CC, O’Brien TP. The role of apoptosis in the early corneal wound healing after excimer laser keratectomy in the rat. Graefe’s archive for clinical and experimental ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2000;238(10):853-60.
5. Wilson SE, Kim WJ. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998;39(2):220-6.
6. Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45(8):2760-6.
7. Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome for LASIK: a randomized controlled study. Ophthalmology. 2007;114(8):1482-90.
8. Santhiago MR, Wilson SE. Cellular effects after laser in situ keratomileusis flap formation with femtosecond lasers: a review. Cornea. 2012;31(2):198-205.
9. Lubatschowski H. Overview of commercially available femtosecond lasers in refractive surgery. J Refract Surg. 2008;24(1):S102-7.
10. Malta JB, Soong HK, Shtein R, Banitt M, Musch DC, Sugar A, et al. Femtosecond laser-assisted keratoplasty: laboratory studies in eye bank eyes. Current Eye Res. 2009;34(1):18-25.
11. Pietila J, Huhtala A, Jaaskelainen M, Jylli J, Makinen P, Uusitalo H. LASIK flap creation with the Ziemer femtosecond laser in 787 consecutive eyes. J Refract Surg. 2010;26(1):7-16.
12. Knorz MC, Vossmerbaeumer U. Comparison of flap adhesion strength using the Amadeus microkeratome and the IntraLase iFS femtosecond laser in rabbits. J Refract Surg. 2008;24(9):875-8.
13. Riau AK, Liu YC, Lwin NC, Ang HP, Tan NY, Yam GH, et al. Comparative study of nJ- and muJ-energy level femtosecond lasers: evaluation of flap adhesion strength, stromal bed quality, and tissue responses. Invest Ophthalmol Vis Sci. 2014;55(5):3186-94.
14. Ahn H, Kim JK, Kim CK, Han GH, Seo KY, Kim EK, et al. Comparison of laser in situ keratomileusis flaps created by 3 femtosecond lasers and a microkeratome. J Cataract Refract Surg. 2011;37(2):349-57.
15. Zhou Y, Tian L, Wang N, Dougherty PJ. Anterior segment optical coherence tomography measurement of LASIK flaps: femtosecond laser vs microkeratome. J Refract Surg. 2011;27(6):408-16.
16. Podesta F, Romeo G, Liu WH, Krajewski S, Reed JC, Gerhardinger C, et al. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol. 2000;156(3):1025-32.
17. Soong HK, Malta JB. Femtosecond lasers in ophthalmology. Am J Ophthalmol. 2009;147(2):189-97 e2.
18. Sarayba MA, Maguen E, Salz J, Rabinowitz Y, Ignacio TS. Femtosecond laser keratome creation of partial thickness donor corneal buttons for lamellar keratoplasty. J Refract Surg. 2007;23(1):58-65.
19. Solomon KD, Donnenfeld E, Sandoval HP, Al Sarraf O, Kasper TJ, Holzer MP, et al. Flap thickness accuracy: comparison of 6 microkeratome models. J Cataract Refract Surg. 2004;30(5):964-77.
20. Paschalis EI, Labiris G, Aristeidou AP, Foudoulakis NC, Koukoula SC, Kozobolis VP. Laser in situ keratomileusis flap-thickness predictability with a pendular microkeratome. J Cataract Refract Surg. 2011;37(12):2160-6.
21. Farjo AA, Sugar A, Schallhorn SC, Majmudar PA, Tanzer DJ, Trattler WB, et al. Femtosecond lasers for LASIK flap creation: a report by the American Academy of Ophthalmology. Ophthalmology. 2013; 120(3):e5-e20.
22. Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411-33.
23. Kim JY, Kim MJ, Kim TI, Choi HJ, Pak JH, Tchah H. A femtosecond laser creates a stronger flap than a mechanical microkeratome. Invest Ophthalmol Vis Sci. 2006;47(2):599-604.
24. de Medeiros FW, Kaur H, Agrawal V, Chaurasia SS, Hammel J, Dupps WJ, Jr., et al. Effect of femtosecond laser energy level on corneal stromal cell death and inflammation. J Refract Surg. 2009;25(10):869-74.
25. Wilson SE, Li Q, Weng J, Barry-Lane PA, Jester JV, Liang Q, et al. The Fas-Fas ligand system and other modulators of apoptosis in the cornea. Invest Ophthalmol Vis Sci. 1996;37(8):1582-92.
26. Netto MV, Mohan RR, Medeiros FW, Dupps WJ, Jr., Sinha S, Krueger RR, et al. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J Refract Surg. 2007;23(7):667-76.
27. Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004;30(1):26-32.
28. Winkler von Mohrenfels C, Khoramnia R, Salgado J, Wullner C, Donitzky C, Maier M, et al. First clinical results with a new 200 kHz femtosecond laser system. Br J Ophthalmol. 2012;96(6):788-92.
29. Winkler von Mohrenfels C, Khoramnia R, Maier MM, Pfaffl W, Holzlwimmer G, Lohmann C. [Cut quality of a new femtosecond laser system]. Klin Monbl Augenheilkd. 2009;226(6):470-4.
30. Khoramnia R, Salgado JP, Wuellner C, Donitzky C, Lohmann CP, Winkler von Mohrenfels C. Safety, efficacy, predictability and stability of laser in situ keratomileusis (LASIK) with a 1000-Hz scanning spot excimer laser. Acta Ophthalmol. 2012;90(6):508-13.
31. Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21(5):1465-8.
32. Foster JW, Wahlin K, Adams SM, Birk DE, Zack DJ, Chakravarti S. Cornea organoids from human induced pluripotent stem cells. Sci Rep. 2017;7:41286.
Submitted for publication:
October 5, 2017.
Accepted for publication:
February 16, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Approved by the following research ethics committee: Hospital Oftalmológico de Sorocaba (CAAE: 16421313.8.0000.0088)