

Bianca Gubiani Ferreira1,2; Diane Ruschel Marinho1,3; Felipe Teloken Diligenti3
DOI: 10.5935/0004-2749.20180063
ABSTRACT
Purpose: To evaluate the response to sub-Tenon’s triamcinolone injection in patients with uveitis.
Methods: We studied 28 eyes with macular edema associated with controlled uveitis. We administered sub-Tenon’s injection of triamcinolone and followed the patients for 180 days to analyze the positive effects (improvement of macular edema and visual acuity) and monitor the possible adverse effects. This prospective study was conducted at the Department of Ophthalmology, Hospital de Clínicas de Porto Alegre, Brazil.
Results: We observed improvement in macular edema in 86% of patients. The mean central macular thickness at each time point of assessment was 432.22, 298.80, 286.37, 267.49, 253.87, and 253.49 µm at baseline (before sub-Tenon’s injection of triamcinolone), 15 days after the procedure, at 30 days, at 60 days, at 90 days, and at 180 days, respectively. The mean reduction in retinal thickness was 30.8%, 33.7%, 38.11%, 41.2%, and 41.35% at 15, 30, 60, 90, and 180 days of follow-up, respectively. Visual acuity also improved in 85.7% of patients, with a mean improvement of 1.36, 1.93, 2.23, 2.26, and 2.30 lines gained on the Early Treatment Diabetic Retinopathy Study chart at 15, 30, 60, 90, and 180 days of follow-up, respectively. No statistically significant increases in intraocular pressure and conjunctival abnormalities were caused by the procedure, and no other adverse effects were observed. Overall, the results of this study were similar to those described in the literature.
Conclusions: Sub-Tenon’s injection of triamcinolone provides reduced macular thickness and improvement in visual acuity with no significant adverse effects and is therefore an effective and safe procedure for the treatment of sequelae of uveitis.
Keywords: Uveitis; Triamcinolone; Intravitreal injections; Macular edema; Tenon capsule; Visual acuity
RESUMO
Objetivos: Avaliar os efeitos da injeção subtenoniana de triancinolona em pacientes com uveítes.
Métodos: Foram incluídos na avaliação 28 olhos com edema macular associado à uveíte. Esses pacientes foram submetidos à injeção subtenoniana de triancinolona e acompanhados ao longo de 180 dias, para analisar os efeitos em relação à melhora do edema macular, da acuidade visual e acompanhamento de possíveis efeitos adversos. Trata-se de um estudo prospectivo, realizado no Serviço de Oftalmologia do Hospital de Clínicas de Porto Alegre.
Resultados: Foi verificada melhora do edema macular em 86% dos pacientes, sendo uma redução média da espessura retiniana de 30,8% aos 15 dias, 33,7% aos 30 dias, 38,11% aos 60 dias, 41,2% aos 90 dias e 41,35% aos 180 dias de seguimento. Também foi observado melhora da acuidade visual em 85,7% dos pacientes e ganho de linhas na tabela de acuidade visual, sendo 1,36 linhas aos 15 dias de seguimento, 1,93 linhas aos 30 dias, 2,23 linhas aos 60 dias, 2,26 linhas aos 90 dias e 2,30 linhas aos 180 dias. Não houve significância estatística em relação ao aumento da pressão intraocular e às alterações conjuntivais causadas pelo procedimento, sem detecção de qualquer outro efeito colateral. Foi concluído que os resultados encontrados nesse estudo são similares aos descritos na literatura.
Conclusões: A injeção subtenoniana de triancinolona é um procedimento eficaz e seguro para o tratamento das sequelas por quadros de uveítes, proporcionando redução da espessura macular e melhora da acuidade visual, sem relação com efeitos adversos significativos.
Descritores: Uveíte; Triancinolona; Injeções intravítreas; Edema macular; Cápsula de Tenon; Acuidade visual
INTRODUCTION
The term “uveitis” refers to any intraocular inflammation affecting the uveal tract(1). Ocular involvement may lead to complications such as cataract, glaucoma, and macular edema-a late complication that, if left untreated for long, may result in irreversible visual loss(2,3).
The aim of clinical treatment of uveitis is to control the inflammation and prevent ocular sequelae. Uveitis is treated mainly with corticosteroids(4,5). This class of medication represents the first line of therapy in patients with non-infectious uveitis, and its effectiveness has stirred heated debate at uveitis treatment centers(6-8). The effect of triamcinolone in the treatment of macular edema secondary to diabetic or vascular retinopathy has been demonstrated in many studies. Few, however, have assessed its role in uveitis-related cases.
In selected cases of unilateral uveitis, the periocular administration of corticosteroids by, for instance, sub-Tenon’s injection, is indicated either as an adjuvant to other routes of administration or where systemic corticosteroid therapy cannot be administered(4). Patients with infectious uveitis should not be administered periocular injections of corticosteroids in the absence of specific antimicrobial therapy, and corticosteroids should be prescribed with caution to patients with a history of increased intraocular pressure induced by corticosteroids(1).
The main advantage of sub-Tenon’s injection of corticosteroids, compared with other routes, is the large volume of medication it delivers to the ocular tissues for a longer period(9). Additional assurance of anatomically correct administration, improved drug availability, and a lower risk of adverse effects are provided by injection under operating microscope visualization(10).
The rare complications related to sub-Tenon’s injection of triamcinolone include increased intraocular pressure, orbital infection, conjunctival ischemia(11) or ulceration(12), an encapsulated triamcinolone cyst(13), and central serous chorioretinopathy(7). Despite these risks, sub-Tenon’s injection of triamcinolone is considered safe(6). In addition, a reduction in retinal thickness in cases of macular edema secondary to uveitis and improvement in visual acuity are associated with periocular triamcinolone therapy(7,14-16).
In this study, we aim to conduct a prospective evaluation of outcomes and complications arising from sub-Tenon’s injection of triamcinolone in patients with uveitis. Our primary objective is to evaluate the effect of sub-Tenon’s injection of triamcinolone in reducing central macular thickness in the presence of macular edema or subretinal fluid. Our secondary objectives are to assess changes in visual acuity and possible adverse effects, such as increased intraocular pressure and conjunctival abnormalities.
METHODS
Study population
The study sample of 28 eyes of patients with controlled uveitis was drawn from patients treated at the Outpatient Uveitis Clinic of the Department of Ophthalmology, Hospital de Clínicas de Porto Alegre, Brazil, all of whom had established indications for sub-Tenon’s injection of triamcinolone in accordance with ambulatory routine. The effect of injection of triamcinolone is local, affecting only the injected eye; we included in the study both eyes of patients with bilateral macular edema. The study included only patients aged 18 years or older, with macular edema or subretinal fluid in the macular region, based on optical coherence tomography (OCT). We excluded patients who had received periocular or intraocular injection of corticosteroids in the preceding 6 months and patients on chronic systemic corticosteroid therapy (to prevent an additive effect of recent use of these medications); we also excluded patients with significant opacity of the intraocular media (such as a cataract or severe vitreitis)-potentially hindering the assessment of visual acuity-and patients who refused to provide written informed consent.
This prospective study was approved by the Research Ethics Committee of Hospital de Clínicas de Porto Alegre with judgment number 13-0532 and was conducted entirely at the Department of Ophthalmology of the aforementioned hospital.
Patients
Patients who underwent sub-Tenon’s injection of triamcinolone under the guidance of an operating microscope were followed prospectively. Patients with infectious uveitis received specific antimicrobial therapy before periocular administration of corticosteroids. We administered antibiotic treatment 15 days before injection in the case of tuberculosis uveitis and 3 days before in the case of toxoplasmosis uveitis. Patients with syphilis were injected simultaneously with the antibiotic treatment.
The same surgeon performed all procedures and also carried out follow-up and assessment of all patients throughout the study. Briefly, one drop of proxymetacaine hydrochloride induced topical anesthesia, the periocular region was prepped with povidone-iodine, an aseptic operating field was established with sterile drapes, and a blepharostat was put in place. Subconjunctival anesthesia was then induced by injection of 0.3 mL 2% lidocaine without vasoconstrictor into the superior temporal quadrant of the bulbar conjunctiva. With the aid of conjunctival scissors and forceps, this area was then opened and the sub-Tenon’s capsule space dissected (in the superior temporal direction), followed by injection of 1 mL triamcinolone acetonide (40 mg/mL), as posteriorly as possible, with the aid of a 24-gauge Abbocath®. We used a flexible cannula, which follows the curvature of the eyeball, thereby reducing the risk of Tenon’s capsule, conjunctival, or scleral perforation. The procedure is demonstrated in figure 1.
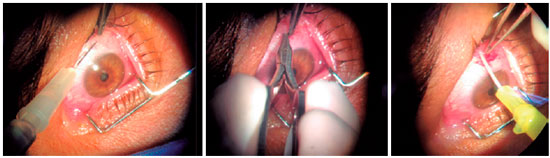
We measured central macular thickness by OCT, performed in a high-definition Cirrus scanner (Cirrus HD-OCT, Carl Zeiss), with images captured in a 512 × 128 Macular Cube. We added the central macular thickness values of all patients and calculated the overall mean for each time point of assessment (before the procedure and 15, 30, 60, 90, and 180 days after the procedure). We then compared pre-procedural values to those measured during follow-up. We considered OCT-measured reductions in macular thickness of at least 100 µm compared with baseline (pre-procedural) values to be relevant.
We measured best-corrected visual acuity using an Early Treatment Diabetic Retinopathy Study (ETDRS) at 4 m (13 ft), scored as the logarithm of the minimum angle of resolution (logMAR). We added the visual acuity values of all patients and calculated the overall mean for each time point of assessment (before the procedure and 15, 30, 60, 90, and 180 days after the procedure). We also calculated the overall mean gain in ETDRS chart lines for all patients at each time point of assessment. We then compared the baseline (pre-procedural) values for both parameters (visual acuity and ETDRS chart lines gained) with those obtained during follow-up.
We measured intraocular pressure with a Perkins tonometer. We added the intraocular pressure values of all eyes and the overall mean calculated for each time point of assessment (before the procedure and 15, 30, 60, 90, and 180 days after the procedure). We then compared pre-procedural values with those measured during follow-up.
We also evaluated adverse effects, such as conjunctival injury (presence of a conjunctival defect at the site of injection on slit-lamp biomicroscopy). We added the conjunctival defect area values of all patients and calculated and compared the overall mean for each time point (15, 30, 60, 90, and 180 days after the procedure). We also assessed the time to resolution of the conjunctival defect.
Statistical analysis
We calculated sample size in WINPEPI version 11.25. On the basis of the findings of a previous study by Adan et al.(17), which found a standard deviation of 66.5 µm before and 143.2 µm after the procedure, and considering a pre-post difference of 100 µm, 90% statistical power, a significance level of 0.05, and a correlation coefficient of 0.5, we estimated the minimum sample size to be 19 eyes.
We expressed quantitative variables as mean (standard deviation) or median and range of values (minimum and maximum) as appropriate, and we expressed qualitative variables as absolute and relative frequencies, using the generalized estimating equations method. We carried out all analyses in PASW Statistics, Version 18.0 (SPSS Inc.) at a significance level of 0.05.
RESULTS
The study, with a total of 19 patients, included 28 eyes-17 (60%) from women and 11 (40%) from men; 15 (53.5%) right eyes and 13 (46.5%) left eyes. The mean age of patients was 45 years (range, 21-74 years). Uveitis in the patients participating in the study had etiologies including seven cases (25%) of tuberculosis, four (14%) of toxoplasmosis, four (14%) of Behçet’s disease, three (11%) of sarcoidosis, three (11%) of idiopathic intermediate uveitis, three (11%) of traumatic uveitis, two (7%) of idiopathic anterior uveitis, and one each (3.5%) of syphilis and psoriatic arthritis.
Among the eyes examined, we considered four (14%) to be unresponsive to treatment, as there was no reduction in retinal thickness at a minimum of two time points of assessment, indicating other modes of treatment in such cases. We included these patients in calculations of results while they remained in the study and excluded them from the sample upon initiation of treatment other than the study intervention. Of these, two eyes with tuberculous uveitis were indicated for other treatment 30 days after the procedure; one eye with uveitis secondary to Behçet’s disease was deemed unresponsive after 30 days; and one eye with traumatic uveitis after 60 days.
In 86% of cases (24 eyes), central macular thickness improved progressively over time. The mean central macular thickness at each time point of assessment was: 432.22, 298.80, 267.49, 253.87, and 253.49 µm at baseline (before sub-Tenon’s injection of triamcinolone), 15 days after the procedure, at 30 days, at 60 days, at 90 days, and at 180 days, respectively. Results are shown in table 1 and figure 2.
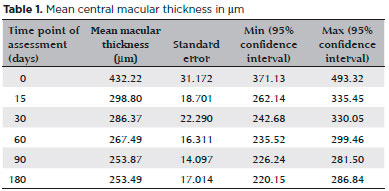
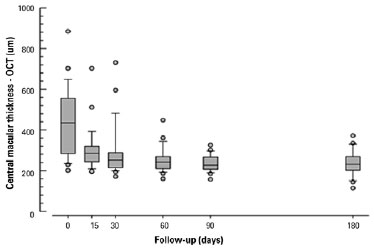
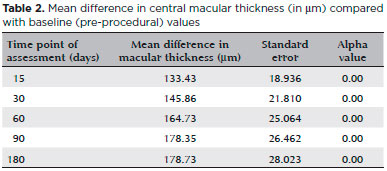
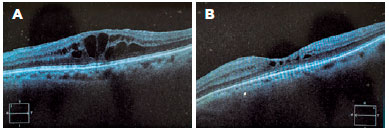
The difference in mean central macular thickness increased at all time points of assessment compared with the baseline measurement (before sub-Tenon’s injection of triamcinolone). The mean reduction in central macular thickness was 133.43 µm (30.8%), 145.86 µm (33.7%), 164.73 µm (38.11%), 178.35 µm (41.2%), and 178.73 µm (41.35%) after 15 days, after 30 days, after 60 days, after 90 days, and after 180 days, respectively. All values were statistically significant (alpha <0.05). Results are shown in table 2. Figure 3 shows pre- and post-treatment OCT findings in one of the patients in our study.
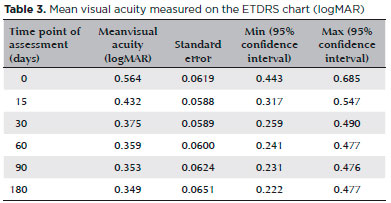
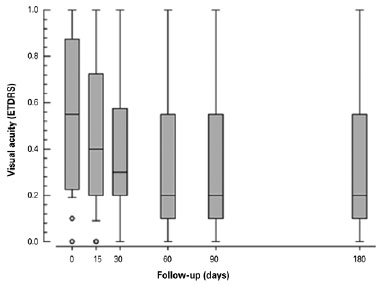
Visual acuity measurements also improved at all time points from baseline. On the ETDRS chart, measures of visual acuity range from 0 to 1, with 0 corresponding to the ability to read the smallest letters (best recorded visual acuity) and 1 corresponding to ability to read only the largest letters (worst recorded visual acuity). The mean ETDRS chart visual acuity (logMAR) was 0.564, 0.432, 0.375, 0.359, 0.353, and 0.349 before sub-Tenon’s injection of triamcinolone, at 15 days after injection, at 30 days, at 60 days, at 90 days, and at 180 days post-procedure, respectively. Results are shown in table 3 and figure 4.
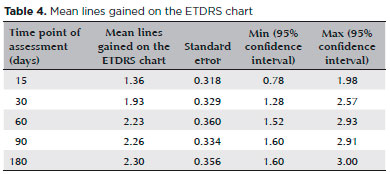
The number of lines gained on the ETDRS chart was progressively greater at all time points of assessment when compared with the baseline (before triamcinolone injection). On average, patients had gained 1.36 lines of acuity, 1.93, 2.23, 2.26, and 2.30 lines at 15, 30, 60, 90, and 180 days after the procedure, respectively. Results are shown in table 4.
There was no statistically significant increase in intraocular pressure from baseline at any time point during follow-up. The mean intraocular pressure was 10.57, 11.43, 13.07, 13.61, 12.32, and 12.24 mmHg prior to sub-Tenon’s injection of triamcinolone 15, 30, 60, 90, and 180 days after the procedure, respectively. Only three patients (10.7%) developed ocular hypertension requiring hypotensive eye drops. The measurements of increased intraocular pressure occurred at 30 days (26 mmHg), 60 days (44 mmHg), and 180 days (34 mmHg). These patients with increased intraocular pressure were treated with hypotensive eye drops for, respectively, 20, 15, and 18 days.
We observed migration of the sub-Tenon’s triamcinolone deposit to the inferior region around the site of injection in only two of 28 eyes (7%), without statistical significance. In these two patients, on slit-lamp examination, the deposit remained visible until 90 days of follow-up but had been reabsorbed and was no longer visible at 180 days.
On slit-lamp examination, the visualized conjunctival defect at the injection site had a mean area of 1.14 mm² at 15 days and 0.32 mm² at 30 days. At 60 day assessment, we were unable to detect any defect in any eye. There was no statistically significant difference in the area of conjunctival defect between time points.
DISCUSSION
In the treatment of complications of uveitis, such as macular edema and low visual acuity, sub-Tenon’s injection of triamcinolone is a relatively simple procedure yielding good outcomes.
In this study, we observed that the administration of triamcinolone to patients with uveitis provides a significant reduction in central macular thickness and improves visual acuity. These effects were appreciable and sustained over a 6 month follow-up period, without any serious adverse effects. Our results were consistent with the findings of previous studies published in the literature.
Reduction in central macular thickness was progressive over 6 months, demonstrating the persistence of the effect of triamcinolone over time. Furthermore, this reduction in macular thickness was detectable on first assessment, 15 days after injection, and persisted at 6 month follow-up. In addition, the deposition of the corticosteroid in the sub-Tenon’s region was visible up to 90 days after the procedure in eyes in which triamcinolone migrated inferiorly to the site of injection. This demonstrates the ability of triamcinolone acetonide to form a deposit, resulting in prolonged effects.
In patients with uveitis and macular edema, visual acuity is also improved with sub-Tenon’s injection of triamcinolone. Mean visual acuity, already improved at first assessment (15 days after injection), continued to increase progressively thereafter over the 6 month follow-up period. This beneficial effect on visual acuity was also evident through the number of lines gained, which also increased progressively at each time point of assessment.
In this study, adverse effects-such as increased intraocular pressure and conjunctival abnormalities-were not significantly prevalent. This indicates that sub-Tenon’s injection of triamcinolone is a safe procedure, if performed with the proper technique and with periodic patient follow-up.
In a retrospective case series, Liu et al. evaluated 37 eyes with uveitis-associated macular edema over a 6 month period. One month after subconjunctival injection of triamcinolone, the mean central macular thickness had decreased from 498.9 µm at baseline assessment to 392.4 µm. Visual acuity had also improved in 54.1% of cases, from an average of 0.45 logMAR on initial assessment to 0.33 logMAR at the end of the study. An increased intraocular pressure (>21 mmHg) occurred in nine eyes (24.3%)(13).
In a retrospective study, Bleriot et al. treated 31 eyes with uveitis-associated macular edema with subconjunctival injection of triamcinolone, followed up over 12 months. The mean visual acuity was 0.36, 0.23, and 0.24 logMAR on initial assessment, at 3 month follow-up, and at 12 month follow-up, respectively. The mean central macular thickness measured on OCT was 444 µm at initial assessment and 355 µm at 3 month follow-up. There was no significant increase in intraocular pressure(18).
In a 6-month prospective study, Tanner et al. evaluated the effects of sub-Tenon’s injection of triamcinolone in patients with posterior and intermediate uveitis. They observed improvement in visual acuity in 89.2% of cases and increased intraocular pressure in 14%(19).
Yoshikawa et al. performed sub-Tenon’s injection of triamcinolone in 39 eyes with uveitis-associated macular edema. At least two lines in visual acuity were gained in 56.4% of treated eyes. However, there was no improvement in visual acuity in 28.2% of eyes. Only one patient (2.5%) developed glaucoma(20).
In a retrospective study, Okada et al. evaluated 51 eyes of patients with posterior uveitis who had received sub-Tenon’s injections of triamcinolone. Overall, 82% of patients exhibited a reduction in macular edema, and improvements in visual acuity were detected as soon as the first month of follow-up; 27% of patients, however, developed increased intraocular pressure(21).
Sub-Tenon’s injection of triamcinolone is beneficial for patients with complications of uveitis, as this procedure reduces retinal thickness in patients with cystoid macular edema secondary to uveitis. Improvement can be observed as early as 15 days after injection and is sustained for at least 180 days thereafter. In terms of visual acuity, triamcinolone injection allowed progressive improvement in the number of lines gained on the ETDRS chart as well as in mean visual acuity (logMAR); this improvement was perceptible shortly after the procedure and also persisted for at least 6 months.
It is a safe procedure, besides the possibility of intraocular hypertension, and is not associated with severe conjunctival injury.
Our study did not refer to the development of cataracts-a frequent complication-because of the long period of follow-up that would have been necessary.
In short, we found sub-Tenon’s injection of triamcinolone to be an effective and safe procedure for reducing central macular thickness in patients with cystoid macular edema secondary to uveitis and also to improve visual acuity in these patients.
REFERENCES
1. Yanoff M, Duker JS, Augsburger JJ. Ophthalmology. 6th ed. Edinburgh: Mosby Elsevier; 2009.
2. Oréfice F, Santos DV, Oréfice JL. Uveítes. Rio de Janeiro: Cultura Médica; 2011. (CBO- Série Oftalmologia Brasileira).
3. Kanski JJ. Oftalmologia clínica: uma abordagem sistemática. Barcelona: Elsevier España; 2008.
4. Orefice F, Santos DV, Orefice JL. Uveíte clínica e cirúrgica texto e atlas. Rio de Janeiro: Cultura Médica; 2011.
5. Alves MR. Bases da Oftalmologia. Rio de Janeiro: Cultura Médica; 2011. v. 1. (CBO-Série Oftalmologia Brasileira).
6. Tempest-Roe S, Joshi L, Dick AD, Taylor SR. Local therapies for inflammatory eye disease in translation: past, present and future. BMC Ophthalmol. 2013;13(1):39.
7. Taylor SR, Isa H, Joshi L, Lightman S. New developments in corticosteroid therapy for uveitis. Ophthalmologica. 2010;224 Suppl 1:46-53.
8. Vedantham V. Periocular corticosteroid therapy: comments. Br J Ophthalmol. 2004;88(5):724-5.
9. Athanasiadis Y, Tsatsos M, Sharma A, Hossain P. Subconjunctival triamcinolone acetonide in the management of ocular inflammatory disease. J Ocul Pharmacol Ther. 2013;29(6):516-22.
10. Rubinstein A, Hanson RJ, Chen SD, Porter N, Downes SM. Conjunctival ischaemia subsequent to posterior subtenon’s triamcinolone acetonide injection. Eye (Lond). 2006;20(3):388-9.
11. Agrawal S, Agrawal J, Agrawal TP. Conjunctival ulceration following triamcinolone injection. Am J Ophthalmol. 2003;136(3):539-40.
12. Chan CK, Mohamed S, Tang EW, Shanmugam MP, Chan NR, Lam DS. Encapsulated triamcinolone cyst after subtenon injection. Clin Exp Ophthalmol. 2006;34(4):360-2. Comment in: Clin Exp Ophthalmol. 2007;35(2):197-8; author reply 198.
13. Liu X, Wang M, Zhao C, Gao F, Zhang M. [The efficacy and safety of subconjunctival triamcinolone acetonide injections in treatment of uveitic macular edema]. Zhonghua Yan Ke Za Zhi. 2015;51(10):734-8. Chinese.
14. Shin JY, Yu HG. Intravitreal triamcinolone injection for uveitic macular edema: a randomized clinical study. Ocul Immunol Inflamm. 2015;23(6):430-6.
15. Salek SS, Leder HA, Butler NJ, Gan TJ, Dunn JP, Thorne JE. Periocular triamcinolone acetonide injections for control of intraocular inflammation associated with uveitis. Ocul Immunol Inflamm. 2013;21(4):257-63.
16. Leder HA, Jabs DA, Galor A, Dunn JP, Thorne JE. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol. 2011;152(3):441-8 e2.
17. Adan A, Pelegrin L, Rey A, Llorenc V, Mesquida M, Molins B, et al. Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina. 2013;33(7):1435-40.
18. Bleriot A, Couret C, Le Meur G, Lebranchu P, Weber M. [Safety and efficacy of subconjunctival triamcinolone injections in the management of uveitic macular edema: retrospective study of thirty-one cases]. J Fr Ophtalmol. 2014;37(8):599-604. French.
19. Tanner V, Kanski JJ, Frith PA. Posterior sub-Tenon’s triamcinolone injections in the treatment of uveitis. Eye (Lond). 1998;12(Pt 4): 679-85.
20. Yoshikawa K, Kotake S, Ichiishi A, Sasamoto Y, Kosaka S, Matsuda H. Posterior sub-Tenon injections of repository corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol. 1995;39(1):71-6.
21. Okada AA, Wakabayashi T, Morimura Y, Kawahara S, Kojima E, Asano Y, et al. Trans-Tenon’s retrobulbar triamcinolone infusion for the treatment of uveitis. Br J Ophthalmol. 2003;87(8):968-71. Comment in: Br J Ophthalmol. 2004;88(5):724-5. Br J Ophthalmol. 2004;88(8:1102-3.
Submitted for publication:
August 10, 2017.
Accepted for publication:
January 16, 2018.
Approved by the following research ethics committee: Hospital de Clínicas de Porto Alegre (#13-0532)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose