

Maosong Xie; Libin Huang; Guoxing Xu
DOI: 10.5935/0004-2749.20180040
ABSTRACT
Purpose: To evaluate the efficacy of prostaglandin antagonists on blood-retinal barrier breakdown induced by anterior segment intraocular simulated surgery.
Methods: Rats were randomly assigned to a negative control group, model group, nonsteroidal anti-inflammatory drugs prophylactic treatment group, nonsteroidal anti-inflammatory drugs treatment group, corticosteroid prophylactic treatment group, and corticosteroid treatment group. Four hours and 48h after modeling, the concentrations of PGE1, PGE2, and PGF2 α in the aqueous humor and vitreous body of the rat model were visualized using ELISA. The integrity of the blood-retinal barrier was quantitatively measured using Evan’s blue as a tracer.
Results: Four hours after modeling, the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the negative control group and the nonsteroidal anti-inflammatory drugs prophylactic treatment group were significantly lower than those in the model group. The concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the corticosteroid prophylactic treatment group were higher than those in the negative control group and the nonsteroidal anti-inflammatory drugs prophylactic treatment group. Forty-eight hours after modeling, the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the nonsteroidal anti-inflammatory drugs prophylactic treatment group, nonsteroidal anti-inflammatory drugs treatment group, corticosteroid prophylactic treatment group, and corticosteroid treatment group were lower than those in the model group, but higher than those in the negative group. Retinal Evan’s blue leakage in the nonsteroidal anti-inflammatory drugs prophylactic treatment group was higher than that in the negative control group, and lower than those in the nonsteroidal anti-inflammatory drugs treatment group, corticosteroid prophylactic treatment group, corticosteroid treatment group, and model group. Retinal Evan’s blue leakage in the nonsteroidal anti-inflammatory drugs treatment group, corticosteroid prophylactic treatment group, and corticosteroid treatment group were lower than those in the model group.
Conclusions: This study confirms that prostaglandin antagonists can relieve blood-retinal barrier breakdown in a rat model and that nonsteroidal anti-inflammatory drugs prophylactic treatment can achieve better efficacy.
Keywords: blood-retinal barrier; surgery; Prostaglandin antagonists; anti-inflammatory agents, non-steroidal; Adrenal cortex hormones; Anterior eye segment/surgery; Rats
RESUMO
Objetivos: Avaliar a eficácia do antagonista de prostaglandinas no rompimento da barreira hemato-retiniana induzida por cirurgia simulada intraocular do segmento anterior.
Métodos: Os ratos foram divididos aleatoriamente em grupo controle negativo, grupo modelo, grupo de tratamento profilático com drogas anti-inflamatórias não esteroides, grupo de tratamento com anti-inflamatórias não esteroides, grupo de tratamento profilático com corticosteroides e grupo de tratamento com corticosteroides. Quatro e 48h após a modelagem, as concentrações de PGE1, PGE2 e PGF2 α no humor aquoso e no corpo vítreo em modelo em ratos foram detectadas através de Elisa. A integridade da barreira hemato-retiniana foi quantitativamente mensurada utilizando o azul de Evans como marcador.
Resultados: Quatro horas após a modelagem, as concentrações de PGE1, PGE2 e PGF2α no humor aquoso e no corpo vítreo no grupo controle negativo e no grupo de tratamento profilático com anti-inflamatórias não esteroides foram significativamente menores do que as do grupo modelo. As concentrações de PGE1, PGE2 e PGF2α no humor aquoso e no corpo vítreo no grupo de tratamento profilático com corticosteroides foram maiores do que as observadas no grupo controle negativo e no grupo de tratamento profilático com anti-inflamatórias não esteroides. 48h após a modelagem, as concentrações de PGE1, PGE2 e PGF2α no humor aquoso e no corpo vítreo no grupo de tratamento profilático com anti-inflamatórias não esteroides, no grupo de tratamento com anti-inflamatórias não esteroides, no grupo de tratamento profilático com corticosteroides e no grupo de tratamento com corticosteroides foram menores do que as observadas no grupo modelo e maiores que as observadas no grupo negativo. O extravasamento retinal de azul de Evans no grupo de tratamento profilático com anti-inflamatórias não esteroides foi maior que no grupo controle negativo e menor que nos grupos de tratamento com anti-inflamatórias não esteroides, de tratamento profilático com corticosteroides, de tratamento com corticosteroides e no grupo modelo. O extravasamento retinal de azul de Evans observado nos grupos de tratamento com anti-inflamatórias não esteroides, de tratamento profilático com corticosteroides e de tratamento com corticosteroides foi inferior ao observado no grupo modelo.
Conclusões: Este estudo valida que o antagonista das prostaglandinas pode aliviar a ruptura da barreira hemato-retiniana em um modelo em ratos e que o tratamento profilático com anti-inflamatórias não esteroides pode alcançar melhor eficácia.
Descritores: Barreira hematorretiniana; Antagosnistas de prostaglandina; Anti-inflamatórios não esteroides; Corticosteroides; Segmento anterior do olho/cirurgia; Ratos
INTRODUCTION
Postoperative cystoid macular edema (CME) can occur following various types of intraocular surgery, such as cataract(1-5), trabeculectomy(6), and corneal transplantation(7-8). Cataract is the most common cause of blindness worldwide, leading to blindness for 10.8 million people and visual impairment for 35.1 million in 2010(9). Glaucoma is the second most common cause of blindness worldwide, with 2.1 million cases of blindness, and 4.2 million cases of visual impairment resulting from glaucoma in 2010(10). CME occurs due to the accumulation of fluid in the central retina (the macula), and postoperative CME is caused by blood-retina barrier (BRB) destruction of the central retina(11). CME is the most common cause of poor visual outcome or visual distortion following cataract surgery. The baseline incidence of pseudophakic CME was 1.17% in eyes without operative complications, diabetes, or risk factors. The risk was higher in the presence of any diabetic retinopathy, epiretinal membrane, uveitis, capsule rupture with or without vitreous loss, retinal vein occlusion, or retinal detachment repair(1).
At present, the exact pathogenesis of postoperative CME remains unclear and has mostly been speculated from clinical observations(12-13). The expression of inflammatory mediators such as prostaglandins (PGs) and cytokines was found to be high after surgery(11). Most investigators, therefore, presumed that inflammation was the major etiological factor for postoperative CME. The inflammation hypothesis presumes that inflammatory mediators can accumulate in the aqueous humor and destroy the blood-aqueous barrier (BAB). Inflammatory mediators can diffuse directly into the vitreous cavity and destroy the BRB, causing accumulation of fluid in the retina(5,1213).
PGs are the most important inflammatory mediators(11), and cyclooxygenase is the rate-limiting enzyme for the synthesis of PGs. nonsteroidal anti-inflammatory drugs (NSAIDs) are nonspecific inhibitors of cyclooxygenase(14) whereas corticosteroids are phospholipase A2 inhibitors(15). Topical and periocular corticosteroids and NSAIDs are the primary methods for the prevention and treatment of CME. Sivaprasad et al.(16) performed a Cochrane review on NSAIDs for treating CME following cataract surgery. However, no conclusion could be safely drawn since the authors commented that many of the trials lacked placebo controls. Sacchi(17) concluded in a review that NSAIDs were considered first-line therapy but there was no evidence-based treatment. Lim(13) also concluded following a review that using topical NSAIDs or steroids to reduce the risk of poor visual outcome after cataract surgery was uncertain.
We established a practical rat model of anterior segment intraocular simulated surgery that induced BRB breakdown(18). The aim of this study was to evaluate the efficacy of PG antagonists on BRB breakdown induced by anterior segment intraocular simulated surgery.
METHODS
Rat model of BRB breakdown induced by anterior segment intraocular simulated surgery
Healthy male Sprague-Dawley rats with clear ocular media (weight 300 ± 20 g, age 8 weeks, Shanghai SLAC Laboratory Animal Co. Ltd., China) were used in this study. This study was performed in strict accordance with the principles suggested by the Association for Research in Vision and Ophthalmology for the care and use of laboratory animals. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Permit Number: 2013-YK-105). Rats were weighed and anesthetized with Ketamine hydrochloride/Xylazine hydrochloride solution (Ketamine hydrochloride 60 mg/kg and Xylazine hydrochloride 4.5 mg/kg) (K4138, Sigma-Aldrich, St. Louis, MO). Local anesthesia was achieved with Oxybuprocaine (Benoxil®, Santen, Japan).
All surgical procedures were performed using sterile techniques. The procedure has been previously reported(18). Briefly, under an operating microscope, a bent 27-gauge needle with a sharp end was inserted through the limbus into the anterior chamber and then removed, avoiding the iris, lens, and corneal endothelium. Another bent 27-gauge needle with a blunt end attached to infusion tubing running to a bottle of balanced salt solution was inserted through the incision into the anterior chamber. The pressure of the balanced salt solution was oscillated from 0 to 12 mmHg (within the rat physiological intraocular pressure range), once per second for a total of 30 times. The needle was removed and the anterior chamber was formed. Finally, the eye was exposed to the light of an operating microscope (8000 lux) for 60 min. The contralateral eye was left untreated. Eyes were treated with ofloxacin ophthalmic solution (Tarivid®, Santen, Japan) three times per day after surgery.
Effect of PG antagonist on BRB breakdown in the rat model
Rats were divided randomly into a negative control group, a model group, an NSAIDs prophylactic treatment group, an NSAIDs treatment group, a corticosteroid prophylactic treatment group, and a corticosteroid treatment group. Each group had 24 rats. The negative control group was administrated with physiological salt solution eye drops. The model group was administrated with physiological salt solution eye drops after modeling. The NSAIDs prophylactic treatment group was administrated with Pranoprofen eye drops (Kotobuki Pharmaceutical Co., Ltd.) 2 days before modeling. The NSAIDs treatment group was administrated with Pranoprofen eye drops after modeling. The corticosteroid prophylactic treatment group was administrated with Prednisolone Acetate eye drops (Allergan Pharmaceutical Co., Ltd) 2 days before modeling. The corticosteroid treatment group was administrated with Prednisolone Acetate eye drops after modeling. Four and 48h after modeling, the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and the vitreous body of the rat model were visualized using ELISA. The integrity of the BRB was quantitatively measured using Evan’s blue as a tracer.
Detection of PG concentrations in the aqueous humor and the vitreous body of the rat model
Rat’s eyes were enucleated and frozen at -20°C for 2h. Then the eyes were cut at the corneoscleral limbus. The frozen aqueous humor and the frozen vitreous body were isolated. The concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and the vitreous body were determined using ELISA according to the manufacturer’s protocol for the rat PGE1 ELISA kit (Cat. No.:DE1200, R&D Inc. USA), rat PGE2 ELISA kit (Cat. No.:514010, Cayman Inc. USA), and rat PGF2α ELISA kit (Cat.No.:516011, Cayman Inc. USA).
Quantitative measurement of the integrity of BRB using Evan’s blue as a tracer
The procedure for quantitative measurement of the amount of BRB breakdown has been previously reported(19). Briefly, rats were injected intravenously with Evan’s blue (45 mg/kg, 206334, Sigma-Aldrich, St. Louis, MO, USA). Upon administration of Evan’s blue, the rats visibly turned blue, and this was used as a confirmation that the dye had been taken into the bloodstream. After 2 h, the rats were perfused via the left ventricle at 37°C using citrate-buffered paraformaldehyde (pH 3.5, 1% wt/ vol). The perfusion lasted 2 min at a physiological blood pressure of 120 mmHg. Immediately after perfusion, both eyes were enucleated. Eyes were bisected at the equator, and retinas were removed under an operating microscope. The retinas were completely dried by placing in a Speed-Vac (5h, 60°C) and retinal dry weights were subsequently determined. Then the retinas were crushed in 120 µl formamide (F5786, Sigma-Aldrich, St. Louis, MO) at 70°C for 18 h, to remove the Evan’s blue. The extract was centrifuged with a filter centrifuge tube at 15,000 rpm for 30 min at 4oC to remove retinal debris. The filtrate was subsequently read on a spectrophotometer (Model Du-640, Beckman, Fulletton, CA) at an absorbance of 620 nm, the absorbance maximum for Evan’s blue, and 740 nm, the absorbance minimum. The concentration of dye in the extracts was calculated from a standard curve of Evan’s blue in formamide. The leakage of Evan’s blue in the retina was calculated using the following equation, with the results expressed in µl plasma × g retinal dry wt1·h1.

Evan’s blue dye was prepared by dissolving it in normal saline (30 mg/ml), sonicating it for 5 min in an ultrasonic cleaner (G1125P1T, Laboratory Supplies, Hicksville, NY, USA), and filtering it through a 5 mm filter (Millipore, Bedford, MA, USA).
Statistical analysis
The concentrations of PGE1, PGE2, and PGF2α and the leakage of Evans blue were presented as mean ± SD. The data were evaluated using an ANOVA analysis with an LSD-t test for multiple comparisons, with p<0.05 considered statistically significant.
RESULTS
Four hours after modeling, ELISA showed that the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the negative control group were similar to those of the NSAIDs prophylactic treatment group. The concentrations for both groups were significantly lower than those in the model group. The concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the corticosteroid prophylactic treatment group were higher than those in the negative control group and the NSAIDs prophylactic treatment group, but lower than those in the model group. The concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the NSAIDs treatment group and the corticosteroid treatment group were similar to those in the model group and higher than those in the negative group. The differences among the six groups were statistically significant (Aqueous humor PGE1: F=971.845, p<0.001; PGE2: F=681.727, p<0.001; PGF2α: F=1590.003, p<0.001; vitreous body PGE1: F=52.982, p<0.001; PGE2: F=93.625, p<0.001; PGF2α: F=766.097, p<0.001). Forty-eight hours after modeling, the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the NSAIDs prophylactic treatment group, the NSAIDs treatment group, the corticosteroid prophylactic treatment group and the corticosteroid treatment group were lower than those in the model group, but still higher than those in the negative group. The differences among the six groups were statistically significant (Aqueous humor PGE1: F=260.980, p<0.001; PGE2: F=188.446, p<0.001; PGF2α: F=168.406, p<0.001; vitreous body PGE1: F=308.369, p<0.001; PGE2: F=90.404, p<0.001; PGF2α: F=368.235, p<0.001). The retinal Evan’s blue leakage in the NSAIDs prophylactic treatment group was higher than that in the negative control group but was lower than those in the NSAIDs treatment group, the corticosteroid prophylactic treatment group, the corticosteroid treatment group, and the model group. The retinal Evan’s blue leakage in the NSAIDs treatment group, the corticosteroid prophylactic treatment group, and the corticosteroid treatment group were lower than those in the model group. The differences among the six groups were statistically significant (4h PGE1: F=482.708, p<0.001; 4 h PGE2: F=1115.055, p<0.001; 4 h PGF2α: F=609.309, p=0.001; 48 h PGE1: F=2193.122, p<0.001; 48 h PGE2: F=3267.795, p<0.001; 48 h PGF2α: F=3106.083, p=0.001) (Figures 1 to 3).
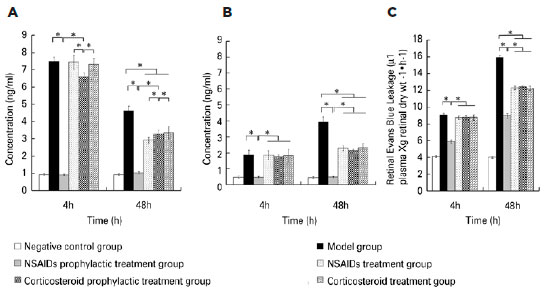
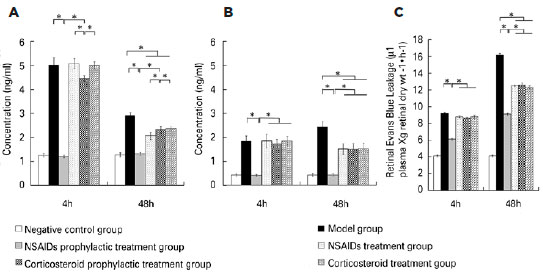
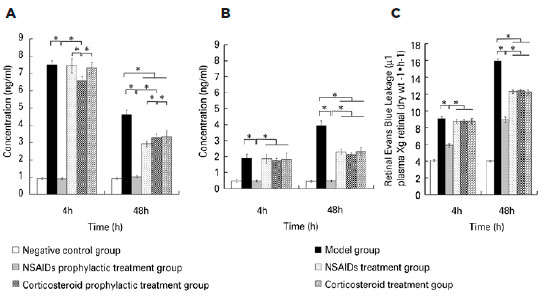
DISCUSSION
We found that the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and the vitreous body in the NSAIDs prophylactic treatment group, the NSAIDs treatment group, the corticosteroid prophylactic treatment group, and the corticosteroid treatment group were lower than those in the model group, but still higher than those in the negative group 48h after modeling. The retinal Evan’s blue leakage in the NSAIDs prophylactic treatment group, the NSAIDs treatment group, the corticosteroid prophylactic treatment group, and the corticosteroid treatment group was lower than that in the model group. PGs are known as inflammatory mediators and can be synthesized in many ocular tissues such as the iris, ciliary body, or lens(20). Surgical procedures can stimulate many ocular tissues, especial the iris, to synthesize PGs. PGs are produced from arachidonic acid by a series of oxidation reactions, whereas arachidonic acid is produced from plasma membrane phospholipids by phospholipase A2. After synthesis, PGs are transported to the extracellular region by the prostaglandin transporter(21-22). PGs are classified into several types including PGA, PGB, PGC, PGD, PGE, PGF, PGG, PGH, and PGI. PGE1, PGE2, and PGF2a couple with their receptors, such as EP1-EP4 and FP, which activates intracellular secondary information through cAMP, IP3, and intracellular signaling cascades. This cross-communication increases the autophosphorylation and dimerization of RTKs, resulting in the activation of mitogen-activated protein kinase or phosphatidylinositol 3-kinase (PI3K) signaling(23-25). Different types of PGs have different functions. For example, PGE can induce inflammation, promote local vasodilation, increase capillary permeability, causing redness, swelling, pain, heat, and other symptoms, whereas PGF2a stimulates smooth muscle contraction and regulates endothelial cell function. PGs stimulate retinal vascular endothelial cells and retinal pigment epithelial cells, increase the permeability of the inner retina barrier and the outer retina barrier, leading to increased retinal Evan’s blue leakage. Corticosteroids are phospholipase A2 inhibitors(15). Cyclooxygenase is the rate-limiting enzyme for PG synthesis. NSAIDs are nonspecific inhibitors of cyclooxygenase(14); therefore, they can prevent inflammation induced by PGs. The synthesis of PGs due to surgical stimulation can be suppressed by pretreatment with NSAIDs(20). As a result, the breakdown of BAB and BRB barriers can also be suppressed.
We found that the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and the vitreous body in the negative control group were similar to those of the NSAIDs prophylactic treatment group 4 h after modeling. Both groups had significantly lower concentrations than those in the model group. This indicated that NSAIDs prophylactic treatment could suppress the synthesis of PGs induced by surgery. The concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and vitreous body in the corticosteroid prophylactic treatment group were higher than those in the negative control group and NSAIDs prophylactic treatment group but lower than those in the model group 4 h after modeling. The retinal Evan’s blue leakage in the NSAIDs prophylactic treatment group was higher than that in the negative control group but was lower than those in the NSAIDs treatment group, the corticosteroid prophylactic treatment group, the corticosteroid treatment group, and the model group. This indicated that the prophylactic treatment effect of NSAIDs was superior to that of corticosteroids. We found that the concentrations of PGE1, PGE2, and PGF2α in the aqueous humor and the vitreous body in the NSAIDs treatment group and the corticosteroid treatment group were higher than those in the NSAIDs prophylactic treatment group 4 h after modeling. This indicated that PG synthesis and secretion is very rapid; thus, NSAIDs prophylactic treatment could achieve better efficacy.
The anterior segment intraocular simulated surgery rat model mimics many features of anterior segment intraocular surgery. In many clinical investigations, clinicians are presented with a set of ill-defined and complicated clinical observations and associated situations, making it difficult to establish a model. The rat lens is spherical, accounting for nearly 4/5 of the volume of the vitreous cavity. This makes it difficult to establish a cataract extraction model because of severe complications and individual differences. To examine pathogenetic mechanisms, investigators frequently select a set of presumed primary factors and proceed to inflict a comparable pathologic insult on animals, looking for clinical manifestations similar to those seen in human patients. Anterior segment intraocular surgery-induced BRB breakdown has been described after cataract extraction, corneal transplantation, and trabeculectomy. Trauma and inflammation, photic injury, and drug toxicity are major presumed pathogenetic mechanisms for BRB breakdown. In this study, trauma and inflammation were mimicked by inserting a needle into the anterior chamber and oscillating while photic injury was mimicked by exposure to the light of an operating microscope. Drug toxicity was induced by applying eye drops. All these procedures could be repeated quantitatively. The rat model manifested the major aspects of BRB breakdown. The leakage was quite severe after surgery as suggested by the extravasation of fluorescein, the increased central retina thickness, strong staining for albumin, and elevated retinal Evan’s blue leakage(18).
In this study, we demonstrated that PG antagonists can relieve BRB breakdown in a rat model and NSAIDs prophylactic treatment can achieve better efficacy.
REFERENCES
1. Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016; 123(2):316-23.
2. Chen XY, Song WJ, Cai HY, Zhao L. Macular edema after cataract surgery in diabetic eyes evaluated by optical coherence tomography. Int J Ophthalmol. 2016;9(1):81-5.
3. Agange N, Mosaed S. Prostaglandin-induced cystoid macular edema following routine cataract extraction. J Ophthalmol. 2010; 2010:690707. 4. Sheppard JD. Topical bromfenac for prevention and treatment of cystoid macular edema following cataract surgery: a review. Clin Ophthalmol. 2016;10:2099-111.
5. Guo S, Patel S, Baumrind B, Johnson K, Levinsohn D, Marcus E, et al. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2015;60(2):123-37.
6. Bui CM, Recchia FM, Recchia CC, Kammer JA. Optical coherence tomography findings in ocular decompression retinopathy. Ophthalmic Surg Lasers Imaging. 2006;37(4):333-5.
7. Oie Y, Nishida K. Triple procedure: cataract extraction, intraocular lens implantation, and corneal graft. Curr Opin Ophthalmol. 2017; 28(1):63-6.
8. Kang JJ, de la Cruz J, Cortina MS. Visual outcomes of Boston keratoprosthesis implantation as the primary penetrating corneal procedure. Cornea. 2012;31(12):1436-40.
9. Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;56(11):6762-9.
10. Bourne RR, Taylor HR, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Number of People Blind or Visually Impaired by Glaucoma worldwide and in world regions 1990 - 2010: A meta-analysis. PLoS One. 2016;11(10):e0162229.
11. Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002;47 Suppl 1:S203-18.
12. Rao SK, Cheung N, Lam DS. Prophylaxis for pseudophakic cystoid macular oedema: a long way to go. Clin Exp Ophthalmol. 2006; 34(4):295-6.
13. Lim BX, Lim CH, Lim DK, Evans JR, Bunce C, Wormald R. Prophylactic nonsteroidal anti-inflammatory drugs for the prevention of macular oedema after cataract surgery. Cochrane Database Syst Rev. 2016;11:CD006683.
14. Rodrigues EB, Farah ME, Bottós JM, Bom AF. Nonsteroidal Anti- inflammatory drugs in the treatment of retinal diseases. Dev Ophthalmol. 2016;55:212-20.
15. Taylor SR, Gurbaxani A, Sallam A, Lightman S. Topical prostaglandin analogues and conjunctival inflammation in uveitic glaucoma. Open Ophthalmol J. 2012;6:75-8.
16. Sivaprasad S, Bunce C, Crosby-Nwaobi R. nonsteroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database Syst Rev. 2012;(2):CD004239.
17. Sacchi M, Villani E, Gilardoni F, Nucci P. Efficacy of intravitreal dexamethasone implant for prostaglandin-induced refractory pseudophakic cystoid macular edema: case report and review of the literature. Clin Ophthalmol. 2014;8:1253-7.
18. Xie MS, Xu GX, Huang Y. Rat model for anterior segment intraocular surgery induced blood-retinal barrier breakdown. Ophthalmologica. 2008;222(1):42-7.
19. Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001; 42(3):789-94.
20. Doucette LP, Walter MA. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2017;38(2): 108-16.
21. Martel-Pelletier J, Pelletier JP, Fahmi H. New insights into prostaglandin biology. J Rheumatol. 2004;31(1):14-6.
22. Leger PL, Pansiot J, Besson V, Palmier B, Renolleau S, Baud O, et al. Cyclooxygenase-2-Derived Prostaglandins Mediate Cerebral Microcirculation in a Juvenile Ischemic Rat Model. Stroke. 2016; 47(12):3048-52.
23. Gao Y, Zhao C, Wang W, Jin R, Li Q, Ge Q, et al. Prostaglandins E2 signal mediated by receptor subtype EP2 promotes IgE production in vivo and contributes to asthma development. Sci Rep. 2016;6:20505.
24. Yun B, Lee H, Jayaraja S, Suram S, Murphy RC, Leslie CC. Prostaglandins from Cytosolic Phospholipase A2α/Cyclooxygenase-1 Pathway and Mitogen-activated Protein Kinases Regulate Gene Expression in Candida albicans-infected Macrophages. J Biol Chem. 2016;291(13):7070-86.
25. Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147-66.
Submitted for publication:
June 1, 2017.
Accepted for publication:
December 19, 2017.
Approved by the following research ethics committee: First Affiliated Hospital of Fujian Medical University (# 2013-YK-105)
Funding: This study was supported by Fujian Provincial Health and Family Planning Commission (grant numbers: 2014-ZQN-ZD-16)
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose