

Karlos Frederico Castelo Branco Sancho1; Claudio Zett1,2; Iran Gonçalves Júnior3; Melina Correia Morales1; Maurício Maia1; Rubens Belfort Mattos Neto1
DOI: 10.5935/0004-2749.20180037
ABSTRACT
Purpose: We aimed to evaluate the effects of oral propranolol for circumscribed choroidal hemangioma.
Methods: In this prospective, longitudinal interventional study, we administered oral propranolol at a dosage of 1.5 mg/kg/day to five patients with circumscribed choroidal hemangioma. We then evaluated visual acuity, binocular indirect ophthalmoscopy, optical coherence tomography, optical coherence tomography angiography, fluorescein and indocyanine green angiography, and ocular ultrasonography at regular intervals and compared changes from the baseline assessments.
Results: No clinical or diagnostic changes were observed in the sizes of the circumscribed choroidal hemangiomas during treatment. Complications due to the hemangioma were reduced in the first four months of treatment, followed by maintenance, before worsening in the subsequent three months.
Conclusions: The study showed that oral propranolol at a dose of 1.5 mg/kg/day did not offer effective monotherapy in the treatment of circumscribed choroidal hemangioma.
Keywords: Hemangioma; Choroid neoplasms; Propranolol; Indocyanine green; Tomography, optical coherence
RESUMO
Objetivo: Avaliar o efeito do propranolol oral para hemangioma circunscrito da coroide.
Métodos: O estudo é do tipo prospectivo, quantitativo e descritivo. Propranolol oral na dose de 1.5 mg/kg/dia foi administrada em cinco pacientes com hemangioma circunscrito da coroide. Todos os pacientes foram avaliados com acuidade visual, oftalmoscopia binocular indireta, tomografia de coerência óptica, angiografia com tomografia de coerência óptica, angiografia com fluoresceína e indocianina verde e ultrassonografia ocular.
Resultados: Nenhuma mudança clínica ou no tamanho do hemangioma circunscrito da coroide foi vista através de métodos diagnósticos em qualquer momento do tratamento. Uma atenuação das complicações foi observada nos primeiros quatro meses de tratamento, com manutenção da condição e piora nos meses seguintes.
Conclusão: O estudo mostrou que o propranolol oral na dose de 1.5 mg/kg/dia não se mostrou efetivo como monoterapia no tratamento do hemangioma circunscrito da coroide.
Descritores: Hemangioma; Neoplasia da coroide; Propranolol; Verde de indocianina; Tomografia de coerência óptica
INTRODUCTION
Circumscribed choroidal hemangioma (CCH) is a rare benign vascular tumor that is usually asymptomatic and diagnosed in adulthood when patients present with reduced visual acuity. It generally causes altered vision that can present as a visual field defects, metamorphopsia, or exudative retinal detachment(1-3). Although noninvasive technologies have improved the diagnosis and follow-up of CCH(4,5), treatment is largely dependent on the tumor location, presence of subretinal fluid, extent of symptoms, and potential for visual recovery. When vision loss is present, treatment options include photodynamic therapy, transpupillary thermotherapy, anti-vascular endothelial growth factor (VEGF), plaque brachytherapy, cryotherapy, external beam radiotherapy, or stereotactic radiation therapy(2,6,7). However, these expensive therapies are unavailable in Brazilian clinics. A few case reports have shown that oral propranolol could be beneficial in the treatment of choroidal hemangioma, but have failed to provide definitive results(8-13). In this study, therefore, we aimed to assess the effects of oral propranolol for the treatment of CCH.
METHODS
This was a prospective longitudinal, interventional study. The research was developed by the Ocular Oncology Department of the Federal University of São Paulo. It was approved by the relevant institutional review board and conducted according to the tenets of the Declaration of Helsinki. Participants were informed of the effects and possible complications of oral propranolol and provided written consent.
We included the first five patients the Ocular Oncology Service admitted with symptomatic CCH in 2016, provided they had not previously received treatment. Disease was considered symptomatic if a patient had low visual acuity, retinal detachment, and exudates. Owing to the rarity of CCH, there was no control group. All participants therefore received oral propranolol at a dosage of 1.5 mg/kg/day. The potential risks of propranolol therapy were managed by monitoring vital signs during follow-up assessments supervised by a cardiology team. All patients were followed up at two weeks and then every month after starting oral treatment. The following test results were documented at each follow-up visit: visual acuity, binocular indirect ophthalmoscopy examination, optical coherence tomography (OCT), OCT angiography, fluorescein and indocyanine green angiography, and ocular ultrasonography. The criterion for discontinuing therapy was any deterioration in the patient’s condition (e.g., worse visual acuity, retinal detachment, or exudates) at two consecutive visits.
Statistical analysis was not performed due to the small cohort. All data are reported descriptively as means (± standard deviations) or ranges, as appropriate.
RESULTS
The mean age of the five participants was 49.6 ± 7.9 years (range: 37-56 years). Among these, two had cystoid retinal degeneration, two had small serous retinal detachments without total macular detachment, and one had total retinal detachment (Figure 1). The B-mode ultrasound showed lesions ranging in height from 3.2 to 4.5 mm (mean: 3.7 mm). No clinical or diagnostic changes were observed in the sizes of the CCHs at any time (Figure 2). At the dose of 1.5 mg/kg/day, all patients experienced a decrease in heart rate on electrocardiography, but none reported any side effects.
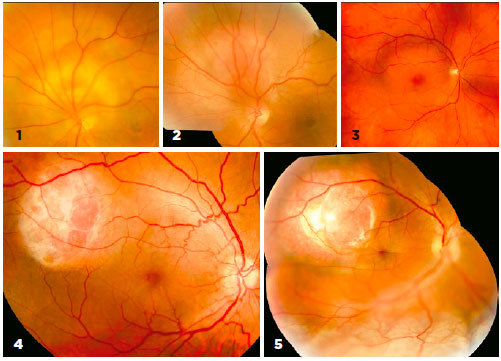
Visual acuity was extremely poor in three cases because of chronic degenerative maculopathy (patients 1, 2, and 5). These patients had suffered low visual acuity for at least one year and were expected to have poor visual outcomes. By contrast, the other two patients (patients 3 and 4) had more recent complaints of visual worsening and metamorphopsia over a few months. These patients had better visual acuity, less metamorphopsia, and smaller serous retinal detachments at the start of treatment (Table 1). By the fourth month of oral β-blocker therapy, however, these patients started to complain of worsening vision and metamorphopsia, although their visual acuity remained objectively unchanged.
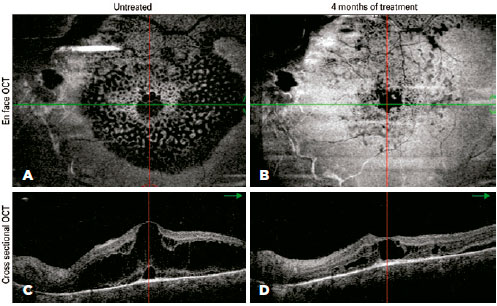
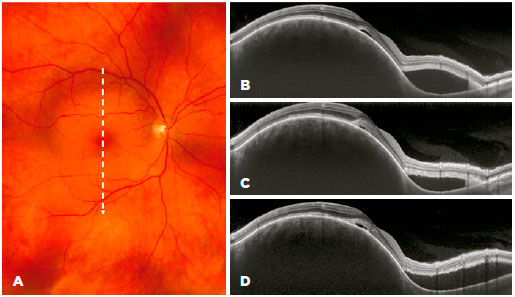
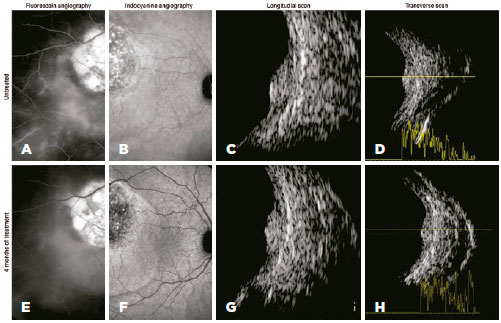
During the first four months of treatment, all patients experienced some anatomical improvement in hemangioma-related complications in the posterior segment (Figure 3). However, in the following three months, the steady sequential improvement was replaced by mild worsening and then clear anatomical worsening (Figure 4), which led to us stopping the β-blocker therapy. Compared with the beginning of treatment, there was no overall deterioration in visual acuity or aggravation of lesions. All patients continue to be followed up, and patients 3 and 4 will be offered alternative therapy if their visual acuities deteriorate.
DISCUSSION
The few case reports on the use of propranolol for choroidal hemangioma have provided insufficient evidence to form definitive recommendations on its use, with some cases showing treatment success and others showing treatment failure(8-13). There is biological plausibility for the efficacy of propranolol, based on increased pericyte-mediated vasoconstriction, inactivation of the renin–angiotensin system, and inhibition of vasculogenesis and catecholamine-induced vasculogenesis (anti-VEGF effect). Although incompletely understood, propranolol is thought to promote its effects on infantile hemangioma through a combination of these mechanisms(14).
In this study, we used oral propranolol to treat symptomatic CCH. Although there was a progressive decrease in the amount of sub- and intraretinal fluid in the first four months of treatment, without full improvement, there was a subsequent stagnation in the therapeutic response followed by worsening despite continued therapy. This behavior raises questions about the mechanism of action of propranolol for this type of tumor, such as whether a saturation point exists. Unfortunately, the lack of a control group means that we cannot know whether the initial improvements resulted from propranolol treatment or natural disease progression. In a case series, oral propranolol therapy was shown to decrease intraretinal and subretinal fluid levels while maintaining the same tumor size(10), but we do not know if this was due to an anti-VEGF effect.
The results of some studies indicate that propranolol only has significant effects with highly concentrated doses (>50 µmol L-1)(15,16). To date, however, no studies have shown the bioavailability of the oral drug in the choroid. Moreover, the only published case of CCH being treated successfully with propranolol reported that a dose of 120 mg/day was used to achieve therapeutic success in one month(13). The research also had two important limitations. First, there was a failure to report the patient’s weight, which precludes calculating the target dose from their data. Second, laser photocoagulation had been done three months before β-blocker therapy was started, and had already produced a partial improvement in the patient’s condition. Thus, we cannot be certain if the improvements resulted from treatment with propranolol or laser photocoagulation.
At a target dosage of 1-3 mg/kg/day, propranolol has been shown to be effective for infantile hemangiomas, leading to suggestions of potential efficacy in the treatment of choroidal hemangiomas. However, hemangiomas do not regress, and may even continue to grow, in approximately 1% of childhood cases, with no satisfactory explanation to account for these cases(17,18). In our clinical study, there was no reduction in CCH size in any patient during treatment with oral propranolol. A hypothesis for this treatment failure is that the choroidal hemangioma presented capillary and cavernous components that differed from those of infantile hemangioma, potentially making them less susceptible to propranolol. Indeed, cavernous vessels are more mature and thicker than capillary vessels(10,12,19).
Oral propranolol at a dose of 1.5 mg/kg/day is ineffective when used as monotherapy for the treatment of CCH in adults. Although CCHs are not malignant, patients with these lesions can develop significant and permanent visual impairments. Further studies should be conducted to help understand this disease and preserve functional vision in affected patients.
REFERENCES
1. Shields JA, Shields CL. Vascular tumors and malformation of the uvea. In: Shields JA, Shields CL. Intraocular tumors: an atlas and textbook, 2nd ed. Baltimore: Lippincott Williams & Wilkins; 2008. p. 230-51.
2. Karimi S, Nourinia R, Mashayekhi A. Circumscribed choroidal hemangioma. J Ophthalmic Vis Res. 2015;10(3):320-8. doi: 10.4103/2008-322X.170353.
3. Berry M, Lucas LJ. Circumscribed choroidal hemangioma: A case report and literature review. J Optom. 2017;10(2):79-83. doi: 10.1016/j.optom.2015.12.006
4. Cennamo G, Romano MR, Breve MA, Velotti N, Reibaldi M, de Crecchio G, et al. Evaluation of choroidal tumors with optical coherence tomography: enhanced depth imaging and OCT-angiography features. Eye (Lond). 2017;31(6):906-15. doi: 10.1038/ eye.2017.14.
5. Balasubramaniam SC, Pellegrini M, Staurenghi G, Pulido JS. Infrared imaging of circumscribed choroidal hemangiomas. Retina. 2017;37(6):1134-9.
6. Tsipursky MS, Golchet PR, Jampol LM. Photodynamic therapy of choroidal hemangioma in Sturge-Weber syndrome, with a review of treatments for diffuse and circumscribed choroidal hemangiomas. Surv Ophthalmol. 2011;56(1):68-85.
7. Naseripour M, Maleki A, Astaraki A, Sedaghat A, Jaberi R, Lee S, et al. Ruthenium-106 brachytherapy in the treatment of circumscribed choroidal hemangioma. Retina. 2017 Mar 23. doi: 10.1097/ IAE.0000000000001616.
8. Arevalo JF, Arias JD, Serrano MA. Oral propranolol for exudative retinal detachment in diffuse choroidal hemangioma [letter]. Arch Ophthalmol. 2011;129(10):1373-5.
9. Dave T, Shah G, Pappuru RR. Diffuse choroidal hemangioma masquerading as central serous chorioretinopathy treated with oral propranolol. Retin Cases Brief Rep. 2016;10(1):11-4.
10. Tanabe H, Sahashi K, Kitano T, Tomita Y, Saito AM, Hirose H. Effects of oral propranolol on circumscribed choroidal hemangioma: A pilot study. JAMA Ophthalmol. 2013;131(12):1617-22. doi:10.1001/ jamaophthalmol.2013.5669.
11. Thapa R, Shields CL. Oral propranolol therapy for management of exudative retinal detachment from diffuse choroidal hemangioma in Sturge-Weber syndrome. Eur J Ophthalmol. 2013;23(6):922-4.
12. Krema H, Yousef YA, Durairaj P, Santiago R. Failure of systemic propranolol therapy for choroidal hemangioma of Sturge-Weber syndrome: a report of 2 cases. JAMA Ophthalmol. 2013;131(5):681-3. doi:10.1001/jamaophthalmol.2013.2879.
13. Sanz-Marco E, Gallego R, Diaz-Llopis M. Oral propranolol for circumscribed choroidal hemangioma. Case Rep Ophthalmol. 2011;25(2):84-90.
14. Ji Y, Chen S, Xu C, Li L, Xiang B. The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. Br J Dermatol. 2015;172(1):24-32. Comment in: Br J Dermatol. 2015;172(1):3-4. Br J Dermatol. 2016;174(2):464.
15. Ji Y, Chen S, Li K, XiaoX, Zheng S. Propranolol: a novel antihemangioma agent with multiple potential mechanisms of action. Ann Surg. 2015;61(2):e52-3. Comment in: Ann Surg. 2015;261(2):e53. Comment on: Ann Surg. 2012;256(1):146-56.
16. Zhang L, Mai HM, Zheng J, Zheng JW, Chen ZG, Wang YA, et al. Preliminary study on plasma RPN concentration of patients with infantile hemangioma treated with propranolol. Int J Clin Exp Med. 2013;6(5):342-5.
17. Caussé S, Aubert H, Saint-Jean M, Puzenat E, Bursztejn AC, Eschard C, Mahé E, Maruani A, Mazereeuw-Hautier J, Dreyfus I, Miquel J, Chiaverini C, Boccara O, Hadj-Rabia S, Stalder JF, Barbarot S; Groupe de Recherche Clinique en Dermatologie Pédiatrique. Propranolol-resistant infantile haemangiomas. Br J Dermatol. 2013; 169(1):125-9.
18. Kumar MG, Coughlin C, Bayliss SJ. Outpatient use of oral propranolol and topical timolol for infantile hemangiomas: survey results and comparison with propranolol consensus statement guidelines. Pediatr Dermatol. 2015;32(2):171-9.
19. Witschel H, Font RL. Hemangioma of the choroid: a clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20(6):415-31.
Submitted for publication:
June 19, 2017.
Accepted for publication:
December 11, 2017.
Approved by the following research ethics committee: Universidade Federal de São Paulo (#1.717.449, CAAE: 57552516.0.0000.5505)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose