

Dimitar Ivanov Grupchev; Mladena Nikolaeva Radeva; Miglena Georgieva; Christina N. Grupcheva
DOI: 10.5935/0004-2749.20180030
ABSTRACT
Purpose: To evaluate microstructural differences between corneas with and without Kayser-Fleischer rings in age-matched subjects with Wilson's disease with neurological symptoms, using confocal laser scanning microscopy.
Methods: The study included 12 subjects with Wilson's disease with neurological symptoms. Twelve corneas presented clinically with classic Kayser-Fleischer rings, visible on slit lamp examination; the other 12 served as controls. The subjects underwent a comprehensive clinical examination. Microstructural analysis using confocal laser scanning microscopy evaluated increased corneal thickness, decreased number of cells, increased debris or specific deposits, and unusual microstructures.
Results: Clinically, the subjects with Kayser-Fleischer rings had similar corneal findings and normal intraocular pressure; two had typical sunflower cataracts and decreased visual acuity. The control eyes all presented normal visual acuity, intraocular pressure, and corneal appearance. The microstructural analysis demonstrated similar findings in all the affected corneas. Compared with the control corneas, there were fewer keratocytes in the anterior stroma (17.380 vs. 22.380/mm3). Round, "hollow" dark areas were observed between the keratocytes; these were universal and similar in appearance in all affected corneas and all cornea layers. In the peripheral posterior stroma, there were dust-like, bright, granular deposits that tended to increase in number and density toward Descemet's membrane, masking the peripheral endothelium. The control corneas presented a normal microstructure apart from dust-like granular deposits in the periphery.
Conclusions: In vivo confocal microscopy is a useful tool for evaluating the corneal microstructure when a Kayser-Fleischer ring is clinically present. The ring consists of granular, bright particles that increase in density toward Descemet's membrane, and is associated with a decreased number of keratocytes and peculiar dark, round areas in all stromal layers, probably a sign of corneal damage. When the ring is not visible in subjects with Wilson's disease, changes to the corneal microstructure are insignificant.
Keywords: Cornea; Hepatolenticular degeneration; Corneal stroma; Descemet membrane; Microscopy, confocal
RESUMO
Objetivo: Avaliar, ao nível microestrutural, através de microscopia confocal in vivo a lazer, 12 córneas com anel de Kayser-Fleischer visível ao exame da lâmpada de fenda e compará-las com 12 córneas clinicamente normais de indivíduos com idades correspondentes aos pacientes com doença de Wilson e sintomas neurológicos.
Métodos: O estudo incluiu 12 indivíduos com doença de Wilson e sintomas neurológicos (24 córneas). Doze córneas apresentavam clinicamente o anel clássico de Kayser-Fleischer e as outras 12 serviram como controle. Todos os pacientes foram submetidos a um exame clínico abrangente e a uma análise microestrutural subsequente utilizando microscopia confocal in vivo de varredura a laser. Os principais resultados observados foram: aumento da espessura da córnea, diminuição do número de células, aumento de resíduos/ depósitos específicos e microestrutura atípica.
Resultados: Clinicamente, todos os indivíduos com anel de Kayser-Fleischer (12 olhos) apresentaram achados similares da córnea e pressão intraocular normal. Dois indivíduos também apresentaram uma catarata de girassol típica e diminuição da acuidade visual. Todos os olhos do grupo controle apresentaram acuidade visual, pressão intraocular e aparência corneana normais. A microscopia confocal in vivo com varredura a laser revelou achados semelhantes em todas as córneas afetadas. O número de ceratócitos no estroma anterior era menor, 17.380/mm3 (22.380/mm3 no grupo controle), e entre eles foram identificadas áreas escuras arredondadas "vazias". Essas zonas escuras eram generalizadas e similares em todas as córneas examinadas e em todas as camadas da córnea. No estroma posterior periférico, havia presença de depósitos granulares brilhantes e com aparência de pó que tendiam a aumentar em número e densidade no sentido da membrana de Descemet, mascarando o endotélio periférico. As córneas controle apresentaram estrutura normal, com exceção de depósitos granulares com aparência de pó na periferia.
Conclusões: A microscopia confocal in vivo é uma ferramenta útil para a avaliação da microestrutura da córnea quando o anel de Kayser-Fleischer está clinicamente presente. O anel é constituído de partículas granulares brilhantes com densidade aumentada no sentido da membrana de Descemet. Sua presença está associada com uma diminuição do número de ceratócitos e com áreas circulares escuras "peculiares" em todas as camadas estromais, que representam, provavelmente, um sinal de dano da córnea. Quando o anel não está clinicamente visível, a estrutura da córnea in vivo encontra-se insignificantemente alterada.
Descritores: Córnea; Degeneração hepatolenticular; Substância própria; Lâmina limitante posterior; Microscopia confocal
INTRODUCTION
Copper is an essential trace element in the human body and a required component of many vital proteins. However, excess copper causes oxidative damage to liver cells, allowing the release of free copper into the blood(1). This blood overload can result in damage to vital organs such as the liver, brain, kidney, and cornea(2). It has been hypothesized that this causes significant toxic damage and is the basis for the clinical consequences of Wilson's disease. This rare disease with autosomal recessive inheritance has significant morbidity and can sometimes be fatal. Recent advances in genetics have identified the gene responsible (ATP7B, located on the 13q14.3 chromosome), and have suggested some options for personalized genetic treatment(3).
Kayser-Fleischer rings are found in 95% of Wilson's disease patients with neurological signs and their presence is considered by some physicians to be important for diagnosis; however, they can also be found in other copper-associated local or systemic pathology(4). Conversely, many patients with Wilson's disease, especially children, do not present any clinically detectable change to the cornea(5). On biomicroscopy, a Kayser-Fleischer ring appears as a brownish-yellow annular deposition of bright, reflective particles at the periphery of Descemet's membrane; it is more prominent on the vertical meridian(6). Although the ring can disappear if the underlying disease is treated appropriately(7), it may be hypothesized, on the basis of the effect of copper accumulations on other target organs such as the liver, kidney, and brain, that the cornea could be permanently damaged, at least at a microstructural level.
There have been few previous reports on the in vivo microstructural characteristics of corneas with clinically observable Kayser-Fleischer rings(8,9). For this reason, we used confocal laser scanning microscopy for a series of patients with clinically diagnosed Wilson's disease and neurological symptoms, comparing the corneal microstructure between the patients with Kayser-Fleischer rings and those with clinically normal corneas. The purpose of the study was to evaluate all corneal layers in detail and describe the microstructural findings associated with the presence of Kayser-Fleischer rings. Signs of corneal toxicity, such as increased corneal thickness, decreased number of cells, increased debris, specific in-morphology deposits, and unusual microstructures, were the primary outcome parameters.
METHODS
The study was conducted at the University Eye Clinic of Varna, Bulgaria, and included 12 subjects (four female and eight male subjects, with a mean age of 25 years). The youngest was a 9-year-old girl, the oldest a 32-year-old man. All had a proven clinical diagnosis of Wilson's disease and had experienced hepatic and neurological symptoms. All had undergone chelation therapy for the previous 6 months. The study was approved by the local ethics committee and was conducted in accordance with the principles of the Declaration of Helsinki. After receiving a detailed explanation of the eye examination and the in vivo confocal technique, the subjects (or their parents) signed an informed consent form.
The 12 subjects were prospectively recruited in two groups: a target group with clinically visible Kayser-Fleischer rings (12 eyes of six subjects, two female and four male, mean age 24 years), and a control group with clinically normal corneas (12 eyes of six subjects, two female and four male, mean age 26 years). No subject had any additional eye disease or history of surgical procedures, and none used contact lenses. The subjects underwent a comprehensive clinical examination, followed by ultrasound pachymetry and in vivo confocal microscopy. The Kayser-Fleisher rings were visualized using slit lamp photography with diffuse illumination and 12× magnification in a completely dark room. Optical sectioning and specular reflection images were also obtained at a higher magnification. The subjects were also examined with a three-mirror Goldmann lens to visualize and take images of the anterior chamber angle; the images stored in the patient's file. Ultrasound pachymetry was performed at the center of the cornea and at four peripheral points (superior, inferior, nasal, and temporal) 2 mm from the corneoscleral junction using a standard pachymeter (OcuScan® RxP Ophthalmic Ultrasound System, Alcon Laboratories, Fort Worth, TX, USA). Confocal microscopy was performed on all the eyes using a Heidelberg Retina Tomograph III confocal laser scanning microscope (Heidelberg Engineering GmbH, Heidelberg, Germany), which is based on a diode laser with a 670 nm wavelength, with the Rostock Corneal Module software version 1.2, following a standard algorithm. Corneregel® fluid (Bausch & Lomb GmbH, Berlin, Germany) was used as a coupling agent between the applanating lens cap and the objective lens. The subject's eyes were anesthetized with topical Alcaine® (0.5%, Alcon Laboratories). After explaining the examination process to the subject, five zones (central, superior, inferior, nasal, and temporal) were examined, following the same procedure for both eyes of all the subjects. The images were stored on a secure server for further analysis.
The analysis was performed by two experienced examiners blinded to the patient data. First, the clinical pictures were classified according to whether or not a Kayser-Fleischer ring was present, and the rings were classified as complete, incomplete, or unusual. The images of the anterior segment were analyzed for lens opacity (the presence or absence of sunflower cataract). Photographic images of the anterior chamber angle were used to distinguish the presence or absence of hyperpigmentation. Blinded to the clinical findings, the examiners qualitatively analyzed the images from the confocal microscopy in volume sections for the five corneal regions (central, superior, inferior, nasal, and temporal), classifying the corneas as normal or abnormal (with descriptions of the microstructural pathology). The pathological observations were subsequently analyzed to unify the description.
RESULTS
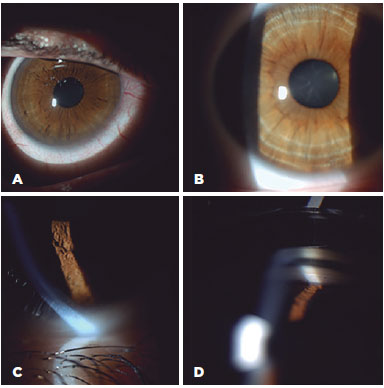
Most of the subjects reported no visual problems (Table 1), with only two of the subjects from the target group having moderately reduced visual acuity in both eyes. The clinically detectable Kayser-Fleisher rings (12 eyes) were all similar in appearance on slit lamp examination; however, those for Subjects 2 and 4 were more prominent on the vertical meridian. Figure 1 illustrates the clinical appearance of Kayser-Fleisher rings on biomicroscopy, including a specular reflection of the left cornea of Subject 4, which showed a localized beaten metal-like appearance, and pigmentation of Schwalbe's line in the right cornea of Subject 5. Subjects 2 and 6 had typical sunflower cataracts; these were the subjects with decreased visual acuity. Intraocular pressure was normal for all the subjects; the maximum difference between the eyes was 2 mmHg in one subject. The results from the comprehensive clinical examination are presented in table 1. The control group demonstrated best-corrected visual acuity ≥1.0 for all eyes and a mean intraocular pressure of 14.75 mmHg. No alteration of the cornea, anterior chamber angle, or lens was clinically detectable by the "blinded" examiners.
Corneal pachymetry of the target group showed the mean thickness of the central cornea to be 585 ± 19, with mean peripheral thicknesses of 680 ± 23 µm superior, 650 ± 24 µm inferior, 660 ± 22 µm nasal, and 662 ± 20 µm temporal. The individual results are presented in table 2. There was no significant difference in thickness between the horizontal and vertical meridians (661 ± 21 µm and 665 ± 23 µm, respectively). In comparison, the corneal measurements for the control group were as follows: central 578 ± 15 µm, superior 630 ± 18 µm, inferior 649 ± 21 µm, nasal 632 ± 22 µm, and temporal 645 ± 21 µm. There was no statistically significant difference between the target and control groups (p=0.1).
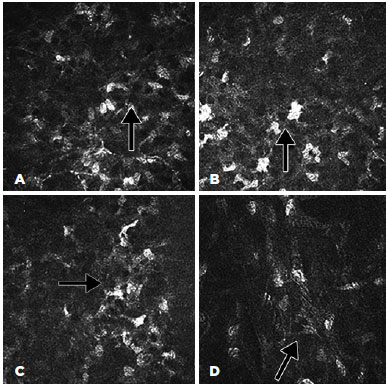
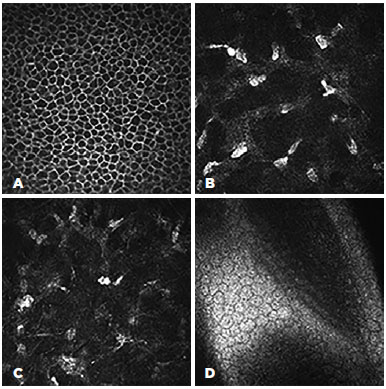
The confocal microscopy demonstrated similar findings for all the subjects from the target group but relatively limited alteration in the corneas of the control group. In the corneas with Kayser-Fleischer rings, the epithelium appeared normal throughout the central and peripheral cornea (Figure 2 A, B). However, there were fewer keratocytes in the anterior stroma than was observed in the control group (17.380 ± 530 vs. 22.380 ± 427/mm3), and there were round, "hollow" dark areas between the keratocytes. Interestingly, these dark zones were universal across all the examined corneas and all cornea layers in the target group (Figure 3 A, B, C). Another unusual finding was that the keratocytes tended to group in the peripheral cornea. There were visibly fewer keratocyte bodies in the posterior stroma than in the anterior stroma (13.520 ± 389 vs. 17.380 ± 530/mm3), and again there were characteristic "hollow" areas (Figure 3 D). In the periphery, close to Descemet's membrane, there were dust-like, bright, granular deposits, which tended to increase in number in the deeper layers (Figure 4). These deposits were so reflective that the endothelium in the periphery was impossible to visualize. In addition to the level of the Descemet's membrane, the deposits were observed within the posterior one-sixth of the stroma. The central endothelium was completely normal (Figure 2 C and D).
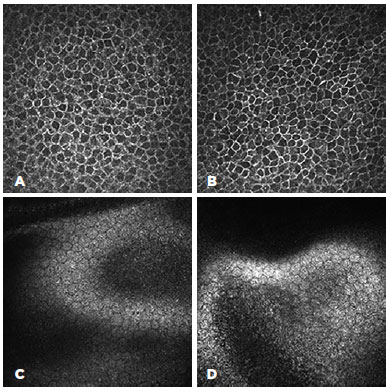
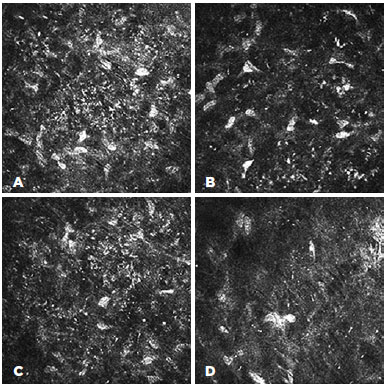
The microstructural analysis of the corneas from the control group (i.e., those without clinically visible Kayser-Fleisher rings) demonstrated normal structures for the epithelium and endothelium (Figure 5 A and D). The corneal stroma, both anterior and posterior, was well populated with keratocytes (22.380 ± 427 and 15.980 ± 412/mm3, respectively), with normal keratocyte morphology (Figure 5 B). The only pathological observation in the control group was the presence of almost accidental minute, bright, granular deposits in the four peripheral quadrants close to, but not limited to, Descemet's membrane (Figure 5 C). However, these were significantly less notable than similar deposits in the corneas of the target group.
DISCUSSION
The Kayser-Fleisher ring appears to be a sign of copper deposition in the cornea. It is widely considered a diagnostic but innocuous finding(10), and it is reversible when treatment reduces the level of copper in the blood stream(11). There have been few clinical observations of the effects of Kayser-Fleisher rings on the corneal microstructure, mainly because of the extremely small number of corneas with copper deposition available for histology. The first report was published in the British Journal of Ophthalmology in 1970(12), in which Harry and Tripathi described fine granular deposition at the level of Descemet's membrane observed on phase contrast microscopy. The deposition zone extended 1.5 mm from the limbus toward the center. Using electron microscopy, they also highlighted "solitary deposits in the posterior stroma", although they considered these to be an accidental finding rather than a universal feature(12).
In our study, we visualized the deposition at the limbus as bright, hyperreflective granules that tended to be more numerous and compact towards Descemet's membrane. These granule-like particles were so hyperreflective that the endothelium was impossible to visualize. However, no endothelial changes were observed in the central cornea. Interestingly, a pathological study by Simonidesova described Hassall-Henle bodies in patients with Kayser-Fleischer rings(13). Our clinical observations were consistent with this finding, but we were unable to visualize these at a microstructural level because of the intensive reflections from the granules. It could be hypothesized that these are not genuine endothelial changes, because the condition is reversible; however, if they are real, Kayser-Fleischer rings cannot be classified as innocuous.
Because of recent therapeutic advances, the survival rate of patients with Wilson's disease has increased significantly. However, some of these patients develop cataracts; if the periphery of their endothelium is damaged, surgical treatment for the cataracts would not be without consequences. In the present study, we demonstrated normal endothelial morphology for the central cornea, which may explain the corneal restoration when the patient's blood copper level returns to within normal range.
In this study, the subjects with visible Kayser-Fleischer rings were matched with subjects with similar systemic presentations but no visible corneal changes. Interestingly, all subjects in the control group presented with deposits but with no other structural changes. A study of patients with Wilson's disease by Ceresara et al. elegantly demonstrated that only 25% of the 20 eyes presented with changes detectable by slit lamp examination, but that confocal laser scanning microscopy revealed deposits in 75% of the eyes(8). The inclusion criteria for Wilson's disease in that study were broader than those for the present study; from the results of the present study, it could be concluded that corneal involvement is universal for Wilson's disease with neurological symptoms. It is possible that the clinical appearance of a Kayser-Fleischer ring is a sign that the corneal stroma is already involved. One question still to be answered is how corneal morphology changes when the clinically visible Kayser-Fleischer ring disappears; this will be the subject of further in vivo confocal morphological studies.
The most recent published study based on in vivo confocal microscopy, by Sturniolo et al., evaluates the epithelium and sub-basal nerve plexus in patients with Wilson's disease(9). It highlighted morphological changes in the epithelium and sub-basal nerve plexus, similar to those in other metabolic diseases, such as a decreased number of fibers, with smaller diameters and increased tortuosity. In the present study, which was also based on in vivo confocal microscopy, the copper depositions were observed as bright granular deposits that started in the posterior one-sixth of the stroma and became denser and more compact towards Descemet's membrane. The most interesting observation, however, was a decreased number of keratocytes and the presence of peculiar "hollow" spaces found in all stromal layers and all observed corneal regions of all of the target group subjects. This was associated with a minimal but detectable increase in corneal thickness compared to that of the control subjects. We can do no more than hypothesize what these "hollow" areas represent. Theoretically, it is possible that these areas may be due to debris from poor cellular function following damage to the mitochondria and Golgi apparatus as a result of the disease process(14). More likely, however, these were areas of apoptotic cells with lost nuclei, which may also explain the decreased number of keratocytes.
This small study included only 12 subjects (24 corneas); however, considering that Wilson's disease is rare and corneal histology is difficult to justify and to obtain tissue for, even postmortem, our results may be useful for better understanding the corneal pathology associated with systemic problems, especially neurological involvement. The observed changes could have serious sight consequences, particularly if the patient has a long life and requires eye surgery. Conversely, the corneal observations described may be useful morphological markers for monitoring the disease.
In conclusion, a Kayser-Fleisher ring is a deposition of copper in the peripheral cornea most commonly associated with Wilson's disease, especially in patients with hepatic or neurological symptoms. This study observed these deposits to be granular, bright particles that increased in density toward Descemet's membrane. Their presence was associated with a decreased number of keratocytes and peculiar dark, round areas in all stromal layers. The central endothelium and epithelium appeared to be unaffected. Further studies are required to assess the significance of these observations and their value as a prognostic marker of disease progress. In vivo confocal microscopy is a useful tool to evaluate corneal microstructure, not only when Kayser-Fleischer rings are clinically present, but also in cases with no corneal involvement detectable by slit lamp examination.
REFERENCES
1. Rosencrantz R, Schilsky M. Wilson disease: pathogenesis and clinical considerations in diagnosis and treatment. Semin Liver Dis. 2011;31(3):245-59.
2. Cocos R, Şendroiu A, Schipor S, Bohîlţea LC, Şendroiu I, Raicu F. Genotype-phenotype correlations in a mountain population community with high prevalence of Wilson's disease: genetic and clinical homogeneity. PLoS One. 2014;9(6):e98520. Erratum in: PLoS One. 2014;9(7):e102619.
3. Lee JY, Kim YH, Kim TW, Oh SY, Kim DS, Shin BS. New novel mutation of the ATP7B gene in a family with Wilson disease. J Neurol Sci. 2012;313(1-2):129-31.
4. Brewer GJ. Neurologically presenting Wilson's disease: epidemiology, pathophysiology and treatment. CNS Drugs. 2005;19(3):185-92.
5. Lin LJ, Wang DX, Ding NN, Lin Y, Jin Y, Zheng CQ. Comprehensive analysis on clinical features of Wilson's disease: an experience over 28 years with 133 cases. Neurol Res. 2014;36(2):157-63.
6. Deguti MM, Tietge UJ, Barbosa ER, Cancado EL. The eye in Wilson's disease: sunflower cataract associated with Kayser-Fleischer ring. J Hepatol. 2002;37(5):700.
7. Marcellini M, Di Ciommo V, Callea F, Devito R, Comparcola D, Sartorelli MR, et al. Treatment of Wilson's disease with zinc from the time of diagnosis in pediatric patients: a single-hospital, 10-year follow-up study. J Lab Clin Med. 2005;145(3):139-43. Erratum in: J Lab Clin Med. 2005;146(1):44.
8. Ceresara G, Fogagnolo P, Zuin M, Zatelli S, Bovet J, Rossetti L. Study of corneal copper deposits in Wilson's disease by in vivo confocal microscopy. Ophthalmologica. 2014;231(3):147-52.
9. Sturniolo GC, Lazzarini D, Bartolo O, Berton M, Leonardi A, Fregona IA, et al. Small fiber peripheral neuropathy in Wilson disease: an in vivo documentation by corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2015;56(2):1390-5.Comment in: Invest Ophthalmol Vis Sci. 2015;56(9):5330. Invest Ophthalmol Vis Sci. 2015;56(9):5331.
10. Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut. 2007;56(1):115-20.
11. Alva-Moncayo E, Castro-Tarin M, Gonzalez-Serrano A. Wilson disease. A case report and review of the literature. Rev Med Inst Mex Seguro Soc. 2011;49(3):331-4.
12. Harry J, Tripathi R. Kayser-Fleischer ring. A pathological study. Br J Ophthalmol. 1970;54(12):794-800.
13. Simonidesova I. [The Kayser-Fleischer ring and its diagnostic importance]. Cesk Slov Oftalmol. 2002;58(3):194-8. Slovak.
14. Kitzberger R, Madl C, Ferenci P. Wilson disease. Metab Brain Dis. 2005;20(4):295-302.
Submitted for publication:
May 24, 2017.
Accepted for publication:
October 16, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Approved by the following research ethics committee: Medical University of Varna (# 54/2016).