

Fernando M. Pradella1; Renato Nisihara2,3; Mario T. Sato1; Alexandre A. Grandinetti1; Sergio B. Novello1; Marcelo Pires1; Diego Schebelski1; Iara Messias-Reason1
DOI: 10.5935/0004-2749.20180027
ABSTRACT
Purpose:To assess whether the serum levels of mannose-binding lectin of the lectin complement pathway are associated with age-related macular degeneration.
Methods: Patients with age-related macular degeneration and age-matched controls underwent full ophthalmologic examination and optical coherence tomography. Using a time-resolved immunofluorometric assay, blood samples were evaluated to determine the serum mannose-binding lectin levels.
Results: A total of 136 individuals were evaluated, including 68 patients with age-related macular degeneration (34 exudative and 34 nonexudative) and 68 age-matched controls. The median mannose-binding lectin level was 608 ng/mL (range, 30-3,415 ng/mL) in patients with age-related macular degeneration and 739 ng/mL (range, 30-6,039 ng/mL) in controls, with no difference between the groups. Additionally, the median mannose-binding lectin level was 476 ng/mL (range, 30-3,415 ng/mL) in exudative cases and 692 ng/mL (range, 30-2,587 ng/mL) in nonexudative cases.
Conclusions: Serum mannose-binding lectin levels were not associated with age-related macular degeneration or with the exudative and nonexudative forms of the disease.
Keywords: ge-related macular degeneration; Drusen; Immunoregulation; Mannose-binding lectin; Inflammation; Retina
RESUMO
Objetivos: Avaliar se as concentrações séricas da lectina ligante de manose da via das lectinas do sistema complemento estão associadas à degeneração macular relacionada à idade.
Métodos: Pacientes com degeneração macular relacionada à idade a controles pareados realizaram exame oftalmológico completo e imagens de tomografia de coerência óptica. As concentrações de lectina ligante de manose foram aferidas em amostras de sangue pelo método "time-resolved Immunofluorometric assay".
Resultados: Um total de 136 indivíduos foram avaliados incluindo 68 com degeneração macular relacionada à idade (34 exsudativa e 34 não-exsudativa) e 68 controles. Concentrações medianas de lectina ligante de ma-nose foram 608 ng/mL (30-3,415 ng/mL) nos casos e 739 ng/mL (30-6,039 ng/mL) nos controles, não havendo diferença entre os grupos. Comparando degeneração macular relacionada a idade exsudativa (mediana de lectina ligante de manose 476 ng/mL; 30-3,415 ng/mL) e não-exsudativa (692 ng/mL; 30-2,587 ng/mL) também não apresentaram diferença.
Conclusões: Concentrações séricas de lectina ligante de manose não estão relacionadas à degeneração macular relacionada a idade ou às formas exsudativa e não-exsudativa.
Descritores: Degeneração macular relacionada à idade; Drusas; Imunoregulação; Lectina de ligação a manose; Inflamação; Retina
INTRODUCTION
Age-related macular degeneration (AMD) is a multifactorial disease with an increasing prevalence worldwide and significant morbidity. Initial studies in the early 1980s indicated the significant burden that AMD would have in the following years. A hereditary polygenic component is one of the main risk factors for the development of AMD; however, a variety of other factors are involved in its pathogenesis, including age (major risk factor), ethnicity, genetics, family history, light exposure, vitamin deficiency, high body mass index (BMI), and smoking(1,2). Cumulative evidence has indicated that inflammation plays a fundamental role in the development of AMD, with the complement system playing a central underlying role in the physiopathological process in addition to the vascular endothelial growth factor (VEGF)(3,4). Serum levels of both VEGF and complement proteins have been found to be increased in AMD. In addition, the special role of the alternative pathway (AP) of the complement system has been described(5). Genetic variants of factors H and B, which are responsible for the inhibition and activation of the AP cascade, respectively, have been shown to be associated with AMD(6,7). In addition to AP, the classic pathway and lectin pathway (LP) of the complement system appear to be necessary for full complement activation and tissue damage in AMD. Joseph et al. showed that in the retinal pigmented epithelial (RPE) cell monolayer, cell lysis does not occur with only AP components, but requires the additional presence of LP components(8). LP is initiated by the binding of pattern recognition molecules, such as mannose-binding lectin (MBL), ficolins, and collectins, to carbohydrates or acetylated residues present on the surface of microorganisms (known as pathogen-associated molecular patterns) or on aberrant glycocalyx patterns of apoptotic, necrotic, or malignant cells(4). MBL is a key protein of LP, which plays a pivotal role in innate immunity and participates in a myriad of biological processes, including carcinogenesis, atherogenesis, and clearance of senescent cells. MBL levels are widely heterogeneous in the general population, and the optimal levels of the protein probably change at different ages, as well as in distinct environmental contexts(9). MBL may offer protection against invading microorganisms; however, high MBL levels may cause biological disadvantages in other situations through the aggravation of local and systemic inflammation involving complement activation and modulation of proinflammatory cytokine production(10). In a recent study, diabetic retinopathy was associated with high MBL levels in a Chinese population(11).
In this study, we investigated the role of MBL in AMD, which is a key element in the activation of LP. In addition, we aimed to determine the association between serum MBL levels and the development and clinical features of AMD.
METHODS
This was a case-controlled study conducted according to the Declaration of Helsinki. All patients submitted a written informed consent and were examined by at least one retinal specialist at the Vision Centre of the Federal University of Paraná. The ethics committee of the Clinical Hospital, Federal University of Paraná, in Curitiba, Brazil, approved the study.
Patients and controls
The study included patients with AMD (category 3 non-exudative AMD or late disease and geographic atrophy or exudative AMD) and age-matched controls. Patients and controls were recruited between July 2011 and January 2014, during regularly scheduled ophthalmologic evaluations at the Vision Centre, Department of Ophthalmology, Federal University of Paraná in Curitiba, Brazil. Patients with AMD and controls with the following inclusion criteria were included:
Patients with AMD
1. Age >55 years
2. History of AMD presenting with large confluent soft drusen (>125 µm) in both eyes or one eye with large drusen and the other eye with either geographic atrophy (>360 µm) or choroidal neovascularization
3. No eye disease other than previous cataract with uncomplicated extraction
4. No history of diabetes or uncontrolled systemic arterial hypertension (self-reported use of medication or physician diagnosed)
5. Refractive error (spherical equivalent) ±4.00 diopters
Controls
All the above criteria, except for item 2.
All patients and controls underwent complete ophthalmologic examination (including best-corrected visual acuity using an ETDRS chart) and fundus examination performed by a retinal specialist. Patients who met the study criteria underwent optical coherence tomography (OCT) imaging of both eyes with a specific imaging protocol. Additional fluorescein angiography was performed in some patients with diagnostic doubt (e.g., vascular disease and macular dystrophy).
Imaging protocol
To ensure that the patients and controls had no macular disease, OCT was performed using the protocol of the "University of Wisconsin, School of Medicine and Public Health, Fundus Photograph Reading Center, Non-study Specific Stratus OCT 3)." The following two scan types were performed: fast macular thickness map scan and cross hair scan. All participants underwent a fast macular thickness map scan [centered on the macula, with the retinal boundaries indicated by white lines in the underlying scans (the internal limiting membrane and the retinal pigment epithelium) not containing obvious major errors at the center of the macula] and a 6-mm cross hair scan (centered on the foveal region). A minimal signal strength of 5 was necessary.
Blood samples and MBL serum levels
Venous blood samples were collected from all patients and controls included in the study. The blood samples were allowed to clot and were centrifuged. The obtained serum samples were aliquoted and stored at -80ºC until use. Serum MBL levels were measured using a time-resolved immunofluorometric assay (TRIFMA) as described by Frederiksen et al.(10). In brief, the diluted serum samples were incubated in mannan-coated wells. After washing, the bound MBL was estimated by applying biotinylated anti-MBL, followed (after washing) by europium-labeled streptavidin and time-resolved fluorometry.
Statistical analysis
AMD cases in Curitiba (a city in South Region, Brazil) were estimated using the demographic features of the Brazilian census in 2013. The Kolmogorov-Smirnov and Mann-Whitney tests were used to compare serum MBL levels. Fisher's exact and chi-squared tests were used for single and demographic comparisons (i.e., age, ethnicity, sex, smoking habit, and BMI), respectively. Values <5% were considered significant.
RESULTS
A total of 136 participants were included in this study. The AMD group included 68 participants (63 European descendants and five Afro-descendants; 33 men and 35 women), including 34 with exudative and 34 with nonexudative clinical presentations (Table 1). The control group included 68 participants (63 European descendants and five Afro-descendants; 27 men and 41 women) without identifiable eye disorders. There was no significant difference in age, sex, or BMI between the groups. In the AMD group, 37 (54%) patients had BMI >25 kg/m2, and in the control group, 44 (65%) had BMI >25 kg/m2. Smoking habits were similar in both groups (Table 1).
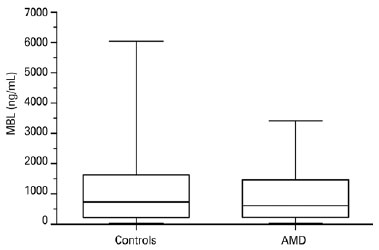
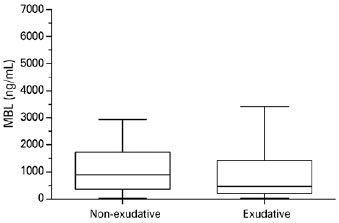
There was no significant difference in serum MBL levels between patients with AMD (median, 608 ng/mL; range, 30-3,415 ng/mL) and controls (median, 739 ng/mL; range, 30-6,039 ng/mL; p=0.1059, Figure 1). Serum MBL levels did not differ between patients with exudative AMD (median, 476 ng/mL; range, 30-3,415 ng/mL) and nonexudative AMD (median, 692 ng/mL; range, 30-2,587 ng/mL; p=0.3291, Figure 2).
Serum MBL levels were divided into the following categories according to the values: low (<100 ng/mL), intermediate (100-2,000 ng/mL), and high (>2,000 ng/mL). The distribution was similar between patients with AMD and controls (low: both groups, 14.71%; intermediate: controls, 45.49% and AMD, 48.53%; high: controls, 39.71% and AMD, 36.76%; p=0.72).
DISCUSSION
The complement system represents a major mechanism in innate host defenses. The three pathways, classic, alternative, and lectin, which can activate the complement system, need to work simultaneously with fine adjustment to maintain homeostasis. Deregulation in complement system activation may be related to the development or aggravation of several diseases, including AMD(12,13). Therefore, a stable and mild MBL level appears to be necessary for a healthy life(9). There is evidence that systemic inflammation is present in AMD, with elements of the complement system (C3, C4, and factor B) found at higher levels in the blood samples of patients with AMD, indicating an inflammatory state not restricted to the retina(14).
The incidence and prevalence of AMD are increasing in different populations, including Brazilian, mainly because of factors such as increased life expectancy, sun exposure, smoking, and obesity. Currently, AMD is the leading cause of vision loss in industrialized countries(15,16).
Several eye diseases, such as glaucoma, diabetic retinopathy, and uveitis, appear to be linked to some type of immune deregulation(17). The activation of the complement cascade by the classical, alternative, or LP, produces inflammatory mediators and leads to target cell damage by the membrane attack complex(17). A low level of complement activation constantly occurs in the eye and is probably needed for protection against pathogens. To regulate and control excessive complement activation that would be damaging to the tissue, ocular fluids and cells express a number of complement regulatory proteins(18). It has been well-established that complement activation has a role in AMD, particularly regarding AP, which is related to polymorphisms of the human factor H gene(19-21). To our knowledge, there is no information on the role of LP in AMD. However, at least in a mouse model, low expression of LP components in retinal tissue was observed and the whole complement system was required for the development of AMD, particularly the exudative form(22,23). Additionally, LP is apparently involved in the lysis of RPE cells in vitro(8). In the present study, serum MBL levels did not differ either between patients with AMD and controls or between exudative and nonexudative forms of AMD. Similar results were found in a study from Australia(24). It is important to emphasize that this Australian study had the same exclusion criteria, AMD definition and plasma MBL levels. The MBL levels obtained for controls were 739 ng/mL in Brazil and 720 ng/mL in Australia (median); the MBL levels in patients with AMD were 608 ng/mL in Brazil and 740 ng/mL in Australia.
LP is initiated when MBL ficolins (L, H, or M) or collectins bind to the microorganism surface (independently of an antibody). Therefore, LP plays an important role in innate immunity. Moreover, MBL/ficolins promote opsonization and phagocytosis of target pathogens. After pathogen binding by MBL/ficolins, MBL-associated serine proteinases (MASP-1 and -2) are activated, followed by complement activation. Structural mutations in the MBL gene may lead to low levels (deficiency) of the protein, thereby increasing susceptibility to infections, particularly at an early age. Conversely, high levels of MBL may be related to inflammatory diseases. For example, patients with retinal herpes infection and endophthalmitis present with high MBL levels(25). Chow et al. evaluated blood and ocular samples (aqueous and/or vitreous) in patients with endophthalmitis and showed that MBL was significantly elevated in inflamed human eyes, but was virtually undetectable in noninflamed eyes, suggesting that MBL has a role in sight-threatening ocular inflammation(25,26).
It is reasonable to consider that a similar immunological environment occurs in AMD, with activation of all complement pathways(27,28). The levels of the components of all pathways of the complement system can increase (in the blood and eyes). However, the relatively high molecular weights of C1q and MBL could be an obstacle for their introduction into retinal circulation. C1q weighs approximately 460 kDa and MBL weighs 450 kDa, whereas factor H weighs only 150 kDa(29). This may explain why no classic or LP molecules were found in drusen(30). A study including intravitreal samples and MBL measures would be able to clarify this point, but such a study would involve invasive examinations, with risks and ethical issues. In diabetic retinopathy, there is a trend toward high MBL levels in the retina and vitreous(11). Vascular abnormalities with a break in the hematorretinian barrier may explain this finding. However, more studies will be necessary for further clarification.
In conclusion, serum MBL levels did not significantly differ between patients with AMD and controls or between exudative and nonexudative forms of AMD. These results suggest that MBL does not play a major role in the development of AMD or in its clinical presentation.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Jens Jensenius from Arhus University, Denmark.
REFERENCES
1. Itty S, Day S, Lyles KW, Stinnett SS, Vajzovic LM, Mruthyunjaya P. Vitamin D deficiency in neovascular versus nonneovascular age-related macular degeneration. Retina. 2014;34(9):1779-86.
2. Baird PN, Chakrabarti S. How genetic studies have advanced our understanding of age-related macular degeneration and their impact on patient care: a review. Clin Experiment Ophthalmol. 2014; 42(1):53-64.
3. Hautamäki A, Kivioja J, Vavuli S, Kakko S, Savolainen ER, Savolainen MJ, et al. Interleukin 8 promoter polymorphism predicts the initial response to bevacizumab treatment for exudative age-related macular degeneration. Retina. 2013;33(9):1815-27.
4. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785-97. Review.
5. Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. In: Lambris JD, Adamis AP, editors. Inflammation and retinal disease: complement biology and pathology. New York: Springer; c2010. p. 9-24. [Advances in Medical and Experimental Biology].
6. Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419-21.
7. Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458-62.
8. Joseph K, Kulik L, Coughlin B, Kunchithapautham K, Bandyopadhyay M, Thiel S, et al. Oxidative stress sensitizes retinal pigmented epithelial (RPE) cells to complement-mediated injury in a natural antibody-, lectin pathway-, and phospholipid epitope-dependent manner. J Biol Chem. 2013;288(18):12753-65.
9. Scorza M, Liguori R, Elce A, Salvatore F, Castaldo G. Biological role of mannose-binding lectin: From newborns to centenarians. Clin Chim Acta. 2015 Dec 7;451(Pt A):78-81.
10. Frederiksen PD, Thiel S, Jensen L, Hansen AG, Matthiesen F, Jensenius JC. Quantification of mannan-binding lectin. J Immunol Methods. 2006;315(1-2):49-60.
11. Geng P, Ding Y, Qiu L, Lu Y. Serum mannose-binding lectin is a strong biomarker of diabetic retinopathy in chinese patients with diabetes. Diabetes Care 2015;38(5):868-75.
12. Smith JR. Immune response and the eye. Clin Experiment Ophthalmol. 2008;36:188.
13. Troutbeck R, Al-Qureshi S, Guymer RH. Therapeutic targeting of the complement system in age-related macular degeneration: a review. Clin Experiment Ophthalmol. 2012;40(1):18-26.
14. Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3(7):1-7.
15. Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-16.
16. Klein R, Chou C, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75-80.
17. Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015;36(6):354-63.
18. Holz FG, Schmitz-valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430-8.
19. Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75-80.
20. Gao YA, Li Y, Xu L, Li Y, Zhang HT, Jonas JB, et al. Complement factor h polymorphism in age-related maculopathy in the Chinese population: The Beijing Eye Study. Retina. 2010;30(3):443-6.
21. Lin JM, Tsai YY, Wan L, Lin HJ, Tsai Y, Lee CC, et al. Complement factor H variant increases the risk for early age-related macular degeneration. Retina. 2008;28(10):1416-20.
22. Luo C, Chen M, Xu H. Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol Vis. 2011;17:1588-97.
23. Rohrer B, Coughlin B, Kunchithapautham K, Long Q, Tomlinson S, Takahashi K, et al. The alternative pathway is required, but not alone sufficient, for retinal pathology in mouse laser-induced choroidal neovascularization. Mol Immunol. 2012;48(6-7): e1-8.
24. Osthoff M, Dean MM, Baird PN, Richardson AJ, Daniell M, Guymer RH, et al. Association study of mannose-binding lectin levels and genetic variants in lectin pathway proteins with susceptibility to age-related macular degeneration: A case-control study. PLoS One. 2015;10(7):e0134107.
25. Chow S, Dean MM, Depla JA, Daniell MD, Eisen DP. Mannose binding lectin as part of the complement pathway: characterization in non inflamed and inflamed human eyes. Clin Exp Ophthalmol. 2011;39(9):871-7.
26. Ulrich JN, Spannagl M, Kampik A, Gandorfer A. Components of the fibrinolytic system in the vitreous body in patients with vitreoretinal disorders. Clin Experiment Ophthalmol. 2008;36(5):431-6.
27. Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2014;134(3):411-31.
28. Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2014;51(2):137-52.
29. Abbas A, Lichtman AH, Pillai S. Cellular and molecular immunology. 8th ed. Philadelphia: Elsevier/Saunders; 2012.
30. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598-614.
Submitted for publication:
June 29, 2017.
Accepted for publication:
August 31, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Approved by the following research ethics committee: Universidade Federal do Paraná (CAAE 0239.0.208.000-11, # 2609.215/2011-09).