

Nelise de Paiva Lucena1; Indira Maria de Sousa Pereira1; Maria Isabel Lynch Gaete1; Kaline Sandrelli Alves Ferreira1; Mathias Violante Mélega2; Rodrigo Pessoa Cavalcanti Lira1,2
DOI: 10.5935/0004-2749.20180022
ABSTRACT
Objective: To study the safety parameters associated with intracameral moxifloxacin application five weeks after cataract surgery.
Methods: The study was a prospective case series set in a private hospital in Recife, Pernambuco, Brazil. A consecutive sample of 1,016 cataract surgeries was evaluated. The inclusion criteria were patients with indications for cataract surgery, a minimum of 55 years of age, and no history of allergy to quinolones. Patients were prepared for surgery using a 5% povidone solution diluted as a topical antiseptic agent. The operative technique was phacoemulsification with intraocular lens implantation. A 0.3mL syringe was partially filled with moxifloxacin and 150 µg/0.03 mL of moxifloxacin was administered through the surgical incision at the end of the surgery. Postoperatively, patients were prescribed: (1) 0.5% moxifloxacin eyedrops 5 times daily for 1 week, and (2) 1% prednisolone acetate eyedrops 5 times daily for 1 week, followed by 4 times daily for 1 week and, subsequently, 2 times daily for 3 weeks. The outcomes were incidence of acute endophthalmitis, mean changes from baseline to 5 postoperative weeks in corneal endothelial cell density, corrected distance visual acuity and intraocular pressure.
Results: The mean age was 67 ± 5 years, and 56.2% of the patients were female. There were no cases of endophthalmitis. The mean preoperative corrected distance visual acuity was 58 letters ± 10 (SD), and the mean postoperative corrected distance visual acuity was 80 letters ± 4 (SD). The mean change in corneal endothelial cell density was 249 cells/mm (10.3%). There was almost no difference in intraocular pressure. No studyrelated adverse events were observed.
Conclusion: The results suggest moxifloxacin is a safe option for intracameral use after cataract surgery.
Keywords: Cataract extraction; Endophthalmitis; Antibiotic prophylaxis; Safety; Postoperative complications
RESUMO
Objetivo: Estudar alguns parâmetros de segurança da moxifloxacino intracameral nas cinco semanas após a cirurgia de catarata.
Métodos: O estudo foi uma série de casos prospectivos. O cenário era um hospital privado em Recife, Pernambuco, Brasil. Foi considerada uma amostra consecutiva de 1.016 cirurgias de catarata. Os critérios de inclusão foram pacientes com indicação para cirurgia de catarata, com pelo menos 55 anos de idade e sem história de alergia a quinolonas. Os pacientes foram preparados para cirurgia usando uma solução de povidona a 5% diluída como agente antiséptico tópico. A técnica operatória foi a facoemulsificação com implante de lente intraocular. Uma seringa de 0,3 mL foi parcialmente preenchida com moxifloxacino. Os pacientes receberam 150 µg/0,03 mL de moxifloxacino através da incisão cirúrgica no final da cirurgia. No pósoperatório, os pacientes foram prescritos: (1) moxifloxacino 0,5% 5 vezes ao dia durante 1 semana e (2) colírio de acetato de prednisolona a 1% 5 vezes ao dia durante 1 semana, seguido de 4 vezes ao dia durante 1 semana e, posteriormente, 2 vezes diariamente por 3 semanas. Os desfechos foram a incidência de endoftalmite aguda, variações entre os valores basais e os na 5ª semana pósoperatória referente à densidade celular endotelial corneana, acuidade visual corrigida para longe e pressão intraocular.
Resultados: A média da idade foi de 67 ± 5 anos, e 56,2% dos pacientes eram do sexo feminino. Não houve casos de endoftalmite. A acuidade visual corrigida para longe préoperatório médio foi de 58 letras ± 10 (SD), e a acuidade visual corrigida para longe pósoperatório médio foi de 80 letras ± 4 (SD). A alteração média na densidade celular endotelial corneana foi de 249 células/mm (10,3%). Não houve diferença na pressão intraocular. Não foram observados eventos adversos relacionados ao estudo.
Conclusão: Os resultados sugerem que o moxifloxacino é uma opção segura para o uso intracameral após a cirurgia de catarata.
Descritores: Extração de catarata; Endoftalmite; Antibioticoprofilaxia; Segurança; Complicações pós-operatórias
INTRODUCTION
The injection of intracameral antibiotics for endophthalmitis prophylaxis has attracted strong interest(1). The use of topical agents alone is less effective and is subject to prescription errors and non-compliance(2). The only commercially available intracameral antibiotic, cefuroxin(3), is unavailable in most countries(1). In addition to its off-label status, it is also unavailable because of dilution risks(4-6). Intracameral moxifloxacin (MFLX) have been used worldwide as an off-label alternative for the prevention of post-cataract endophthalmitis(7,8). It provides broad-spectrum coverage and exhibits concentration- dependent efficacy(9). However, there is little prospective data on its safety(10,11).
The purpose of this study was to determine the safety parameters associated with intracameral MFLX application five weeks after cataract surgery.
METHODS
This study was a prospective study consisting of a consecutive sample of 1016 cataract surgeries at a private hospital in the city of Recife, Pernambuco, Brazil, between 2015 and 2017 (1,016 eyes from 1,016 patients). Ethics committee (institutional review board) approval was obtained, and all participants provided informed consent (National Bioethics Commission of Brazil Identifier No.: 46523115.3.0000.5208). The inclusion criteria were patients with indications for cataract surgery, a minimum of 55 years of age, and no history of allergy to quinolones. Patients with any ocular diseases in addition to cataracts were excluded.
Patients were prepared for surgery using a 5% povidone solution diluted as a topical antiseptic agent. The operative technique was phacoemulsification with intraocular lens implantation. A 0.3-mL syringe was partially filled with MFLX and 150 µg/0.03 mL of MFLX was administered through the surgical incision at the end of the surgery. Postoperatively, patients were prescribed: (1) 0.5% MFLX eyedrops 5 times daily for 1 week, and (2) 1% prednisolone acetate eyedrops 5 times daily for 1 week, followed by 4 times daily for 1 week and, subsequently, 2 times daily for 3 weeks.
The outcomes were incidence of presumed acute postoperative endophthalmitis, mean changes from baseline to 5 weeks after surgery in corneal endothelial cell density (CECD), corrected distance visual acuity (CDVA as per the Early Treatment Diabetic Retinopathy Study letter score), and intraocular pressure (IOP).
Continuous variables were compared using the Wilcoxon Signed Rank test. The p-value was two-tailed for IOP and one-tailed for CDVA and CECD. Statistical significance was set at 0.05.
RESULTS
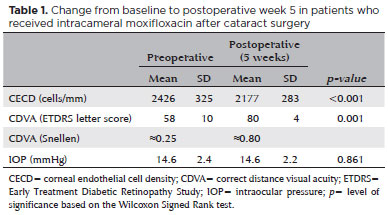
All 1,016 patients (1,016 eyes) completed the study. The mean age was 67 ± 5 years (with a range from 58 to 81 years), and 56.2% (572 patients) were female. There were no cases of endophthalmitis. The mean preoperative CDVA was 58 letters ± 10 (SD), and the mean postoperative CDVA was 80 letters ± 4 (SD). The mean change in CECD was 249 cells/mm (-10.3%). There was almost no difference in IOP. The results of the secondary outcomes (CDVA, IOP, and CECD) are summarized in table 1. No study-related adverse events were observed.
DISCUSSION
The major virtue of this study is that it is the largest genuinely prospective series evaluating the safety profiles of intracameral MFLX in endophthalmitis prophylaxis after cataract surgery. Antibiotics may cause toxicity when used in the irrigating solution or injected intracamerally at the conclusion of cataract surgery(4-6). Many studies have suggested that intracameral MFLX would be a safe option for the prevention of endophthalmitis; however, most of the data is from retrospective studies(7,8,12).
In this prospective study, there were no cases of endophthalmitis. In other large retrospective series, the incidence of endophthalmitis was lower when intracameral MFLX was compared to placebo. For example, in a retrospective analysis of 600,000 surgeries at ten regional Aravind Eye Hospitals, Haripriya et al. observed that intracameral MFLX reduced the overall rate of endophthalmitis by 3.5-fold (three-fold for manual small-incision cataract surgery and nearly six-fold for phacoemulsification)(7). In a retrospective analysis of 18,000 surgeries in Japanese institutions, Matsuara et al. found that intracameral MFLX administration decreased the risk of endophthalmitis by 3-fold(8).
Malik et al. conducted a small trial with 60 eyes to study the effect of intracameral MFLX (500 µg/0.1 mL) versus no injection on the morphology and cell density of the corneal endothelium in cases of phacoemulsification. There were no significant differences in the preoperative and postoperative values for the coefficient of variation in cell area, hexagonality, or corneal thickness in either of the groups six weeks after surgery(10).
Kokterkir et al. found no significant differences in a similar trial with 60 eyes (250 µg/0.05 mL intracameral MFLX injected in 30 eyes) in which CDVA, IOP, corneal, pachymetry, corneal edema, and retinal thickness were evaluated preoperatively and for three days postoperatively(13).
In a prospective randomized combinedcenter openlabel trial, Lane et al. studied 57 eyes from 47 patients treated with intracameral MFLX (250 µg/0.050 mL) or an equal volume of balanced salt solution upon completion of cataract surgery with intraocular lens implantation. Safety parameters, including BCVA, IOP, CECD, corneal pachymetry, corneal edema, anterior chamber cells, and flare, were evaluated preoperatively and for three months postoperatively. They concluded there was no increased safety risk associated with a 250µg/0.050mL intracameral injection of MFLX(14).
Lira et al. compared the last 150 surgeries before and the first 150 surgeries after the introduction of intracameral MFLX for the prevention of post-cataract endophthalmitis. They found no significant mean-change differences between the groups in terms of pachymetry, CDVA, or IOP from baseline to 2 years after surgery(11). Furthermore, in both groups, the mean change in CECD was similar to our results and to the current literature(15,16).
The main limitation of this study is the relatively small sample. Incidences of endophthalmitis typically vary from 0.04% to 0.5%; therefore, the absence of endophthalmitis could have occurred by chance. However, the other safety data results (CDVA, CECD, and IOP) are robust and encourage the routine use of MFLX. Another limitation is that retinal thickness was not evaluated.
Although our results strongly suggest benefits from the use of intracameral MFLX for endophthalmitis prophylaxis in cataract surgery, a randomized masked clinical trial to test this hypothesis is recommended.
REFERENCES
1. Barry P, Cordovés L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions [Internet]. Dublin: European Society of Cataract and Refractive Surgeons; 2013. [cited 2017 Jun 18]; Available from: http://www.sbop.com.br/conteudo/OK%202013%20ESCRS%20Endophthalmitis-Guidelines.pdf
2. Herrinton LJ, Shorstein NH, Paschal JF, Liu L, Contreras R, Winthrop KL, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-94.
3. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-88.
4. Delyfer M-N, Rougier M-B, Leoni S, Zhang Q, Dalbon F, Colin J, et al. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. J Cataract Refract Surg. 2011; 37(2):271-8.
5. Olavi P. Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50mg/ml intracameral cefuroxime. Acta Ophthalmol (Copenh). 2012;90(2):e153-4.
6. Toxic anterior segment syndrome: etiology [Internet]. [cited 2017 June 21]. Available from: https://www.aao.org/focalpointssnippetdetail.aspx?id=35b758bf-71a0-4264-bdb6-62d4a7f5be12
7. Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: analysis of 600 000 surgeries. Ophthalmology. 2017;124(6):768-75.
8. Matsuura K, Miyoshi T, Suto C, Akura J, Inoue Y. Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J Cataract Refract Surg. 2013;39(11):1702-6.
9. Stroman DW, Dajcs JJ, Cupp GA, Schlech BA. In vitro and in vivo potency of moxifloxacin and moxifloxacin ophthalmic solution 0.5%. A new topical fluoroquinolone. Surv Ophthalmol. 2005; 50(6):S16-31.
10. Malik VK, Kapoor R, Malik KPS, Kumar S, Jain C, Jhalani R. Effect of Intracameral Moxifloxacin (0.5mg) on morphology and cell density of corneal endothelium in phacoemulsification surgery. Del J Ophthalmol Soc. 2016;27(2):102-5.
11. Lira RP, de Paiva Lucena N, Ferreira KS, dos Santos BM. Long-term safety of intracameral moxifloxacin after cataract surgery. J Cataract Refract Surg. 2017;43(1):139-40.
12. Vieira IV, Boianovsky C, Saraiva TJ, Godoy RB de, Lake J, Vieira IV, et al. Safety and efficacy of intracameral moxifloxacin injection for prophylaxis of endophthalmitis after phacoemulsification. Arq Bras Oftalmol. 2017;80(3):165-7.
13. Koktekir BE, Aslan BS. Safety of prophylactic intracameral moxifloxacin use in cataract surgery. J Ocul Pharmacol Ther. 2012;28(3): 278-82.
14. Lane SS, Osher RH, Masket S, Belani S. Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg. 2008;34(9):1451-9.
15. Bourne RRA, Minassian DC, Dart JKG, Rosen P, Kaushal S, Wingate N. Effect of cataract surgery on the corneal endothelium. Ophthalmology. 2004;111(4):679-85.
16. Burkhard Dick H, Kohnen T, Jacobi FK, Jacobi KW. Long-term endothelial cell loss following phacoemulsification through a temporal clear corneal incision. J Cataract Refract Surg. 1996;22(1):63-71.
Submitted for publication:
September 12, 2017.
Accepted for publication:
September 24, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Approved by the following research ethics committee: Universidade Federal de Pernambuco Centro de Ciências da Saúde (#1.257.760, 5208).