

Seyhan Dikci; Osman Melih Ceylan; Soner Demirel; Turgut Yılmaz
DOI: 10.5935/0004-2749.20180005
ABSTRACT
Purpose: To compare 0.5 mg and 0.625 mg of bevacizumab for treating aggressive posterior retinopathy of prematurity (AP-ROP).
Methods: The medical records of patients with AP-ROP who were administered intravitreal bevacizumab (IVB) as a primary treatment at a university clinic were evaluated retrospectively. Five eyes of three patients (Group 1) who received 0.625 mg/0.025 ml IVB and 10 eyes of another five patients (Group 2) who received 0.5 mg/0.02 ml IVB were evaluated. Laser photocoagulation was used as additional treatment after relapses. Anatomic results and complications were evaluated in both groups.
Results: We evaluated 15 eyes of eight patients (four girls and four boys) with a flat demarcation line at posterior zone 2 and plus disease or stage-3 disease in this study. The mean gestational age of the three babies in Group 1 was 26 ± 1 weeks and the mean birth weight was 835.33 ± 48.01 g. The corresponding values were 25.2 ± 1.6 weeks and 724 ± 139.03 g, respectively, for the five babies in Group 2. Retinal vascularization was completed at a mean postmenstrual duration of 53.6 ± 1.5 weeks without additional treatment in the five eyes in Group 1. Laser photocoagulation for relapse was administered to five of the 10 eyes in Group 2. Retinal vascularization was completed at a mean postmenstrual duration of 47.6 ± 1.5 weeks in the remaining five eyes. None of the patients developed complications such as cataract, glaucoma, retinal tear, retinal or vitreous hemorrhage, or retinal detachment.
Conclusion: Although lower IVB doses in the treatment of AP-ROP are expected to be safer in terms of local and systemic side effects in premature infants, these patients may require additional treatment with IVB or laser photocoagulation.
Keywords: Retinopathy of prematurity; Bevacizumab/administration & dosage; Light coagulation/methods; Vascular endothelial growth factor/antagonists & inhibitors
RESUMO
Objetivo: Comparar doses de 0,5 mg e 0,625 mg de bevacizumab no tratamento da retinopatia da prematuridade posterior agressiva (ROP-PA).
Métodos: os registros médicos de pacientes com ROP-PA que receberam bevacizumab intravítreo (IVB) como tratamento primário em uma clínica universitária foram avaliados retrospectivamente. Houve 5 olhos de 3 casos (Grupo 1) que receberam 0,625 mg/0,025 ml de IVB e 10 olhos de outros 5 casos (Grupo 2) que receberam 0,5 mg/0,02 ml de IVB. A fotocoagulação com laser foi utilizada como tratamento adicional para casos de recidiva. Os resultados e complicações anatômicas foram avaliados em ambos os grupos.
Resultados: Incluímos os 15 olhos de 8 pacientes (4 meninas e 4 meninos) com linha de demarcação plana na zona posterior 2 e doença "plus" (dilatação e tortuosidade vascular) neste estudo. A idade gestacional média dos três bebês no Grupo 1 foi de 26 ± 1 semana e o peso médio ao nascer foi de 835,33 ± 48,01 g, enquanto esses valores foram de 25,2 ± 1,6 semanas e 724 ± 139,03 g, respectivamente, para os cinco bebês do Grupo 2. A vascularização da retina foi completada com uma duração média pós-menstrual de 53,6 ± 1,5 semanas sem tratamento adicional nos cinco olhos no Grupo 1. A fotocoagulação a laser foi administrada devido à recaída em 5 dos 10 olhos do Grupo 2. A vascularização da retina foi completada em média de 47,6 ± 1,5 semanas do período pós-menstrual nos cinco olhos restantes. Nenhum dos casos desenvolveu complicações, como catarata, glaucoma, rasgo da retina, hemorragia retiniana ou vítrea ou descolamento da retina.
Conclusão: Embora as doses mais baixas de IVB no tratamento de ROP-PA sejam mais seguras em termos de efeitos colaterais locais e sistêmicos em prematuros, esses pacientes podem precisar de tratamento adicional com IVB ou fotocoagulação a laser.
Descritores: Retinopatia da prematuridade; Bevacizumab/administração & dosage; Fotocoagulação/métodos; Fator A de crescimento do endotélio vascular/antagonistas & inibidores
INTRODUCTION
Retinopathy of prematurity (ROP) with inadequate growth and development of retinal blood vessels in premature infants is one of the foremost reasons for childhood blindness(1,2). Aggressive posterior ROP (AP-ROP) is a clinical entity where plus disease or stage-3 disease is present in zone 1 or posterior zone 2 and early treatment is necessary(3). Laser photocoagulation (LPC), where the avascular retina is ablated, is the gold standard treatment for serious ROP, as in threshold and type-1 prethreshold ROP. This suggestion has been made by both the Cryotherapy for ROP Cooperative (Cryo-ROP) Study and the Early Treatment ROP Cooperative Group (ET-ROP) randomized clinical studies. However, the anatomic and functional results of laser or cryotherapy are poor when the disorders affect zone 1 or posterior zone 2(4,5). Severe narrowing of the visual field, macular heterotopia, myopia, and retinal detachment can develop with the destructive method used for conventional (confluent) laser treatment in these cases(4,5). Therefore, there has been a search for new treatments in ROP, and especially in AP-ROP cases.
Inhibitors of vascular endothelial growth factor (VEGF), which play an important role in the pathogenesis, began to be used in AP-ROP treatment once the pathogenesis was better understood. Bevacizumab monotherapy was significantly effective compared to LPC in zone-1 disease in the Bevacizumab Eliminates the Angiogenic Threat of ROP (BEAT-ROP) study. However, the number of cases included in the study was insufficient to evaluate the treatment reliability(6). There is currently no consensus on the optimum dose of anti-VEGF in ROP treatment, which anti-VEGF to use, and how long the cases should be followed up. Furthermore, our current knowledge on issues regarding the long-term systemic and ocular side effects of this treatment is inadequate. AntiVEGFs are effective in ROP at very different doses. In this retrospective study, we aimed to compare the effectiveness of 0.5 mg and 0.625 mg of bevacizumab for AP-ROP treatment.
METHODS
Medical records of 327 premature infants who had an indirect ophthalmoscopic evaluation and were followed up at İnönü University Turgut Özal Medical Center's newborn intensive care unit, or those referred to our clinic, between May 2013 and September 2015 were retrospectively evaluated. Excluded from this study were patients without ROP (n=200), those with low-risk prethreshold ROP (n=101) or high-risk prethreshold ROP (n=17), and one patient who was administered intravitreal bevacizumab (IVB) combined with LPC. LPC was performed on 17 patients with high-risk prethreshold ROP. We included patients (n=8) who received IVB monotherapy primarily with the diagnosis of AP-ROP (Figure 1). This study was conducted in accordance with the Helsinki Declaration. Study approval was obtained from the local ethics committee. An informed consent form was signed by a family member or the guardian of all patients and detailed information on the treatment was provided orally.
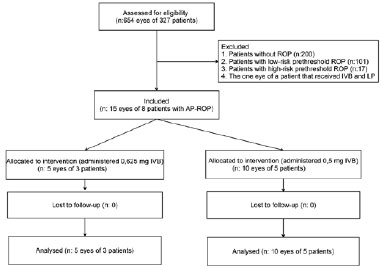
The patients who had plus or stage-3 disease in zone 1 or posterior zone 2, in accordance with ICROP criteria, were classified as having AP-ROP. In our study, all the patients in both groups had plus and stage-1-2 disease in posterior zone 2. The clinical features of all cases were similar. The treatment decision was made according to the criteria defined in the ET-ROP study. The 0.625-mg dose recommended in the BEAT-ROP study was administered to the five eyes of the first three patients (Group 1). This study was not planned in a prospective manner. Rather, the study was planned after the monitoring of 15 eyes from eight patients had been completed. Considering the potential problems with anti-VEGF administration in premature babies, in whom organ and tissue development is ongoing, we reviewed articles reporting that lower doses were effective and decided to administer lower doses during the later part of the study period. Therefore, 0.5 mg of bevacizumab was administered to the 10 eyes of the five patients treated later (Group 2).
The gestational age, birth weight, systemic diseases, retinal vascularization completion times, gestational ages at first treatment and additional treatments, occurrence of relapse, and the number of laser spots administered as additional treatment were evaluated for all patients.
The procedures were conducted with topical anesthesia using 0.5% proparacaine HCl under operating room conditions. After administering 5% povidone iodine (Baticon) to the eyelid and eye and waiting for 1 min, the eye was covered with a sterile drape and a lid speculum was inserted. IVB was administered with a 30-gauge needle 1.5 mm from the upper temporal limbus. A fundus examination was conducted with an indirect ophthalmoscope and a 28-D lens. The central retinal artery and the lens were evaluated in addition to whether a retinal tear was present. Moxifloxacin drops were administered four times a day for 7 days after the procedure. The fundus examination was repeated on the first day, fourth day, first week, first month, and until retinal vascularization was completed, if required. Confluent argon LPC was preferred to a second intravitreal injection in the event of a relapse. Relapse was defined as an increase in the tortuosity of the arteries with venous dilation, development of retinal neovascularization, and a fibrovascular membrane extending into the vitreous.
The follow-up examinations of the patients and their treatment, including an IVB injection and LPC, were performed by the same ophthalmologist (Dr. S.D.). Treatment decisions were made by two ophthalmologists (Dr. S.D. and Dr. T.Y.) who were experienced in the diagnosis of the disorder. Statistical analyses were performed with SPSS for Windows version 17.0 program. Continuous data were reported as mean ± standard deviation (SD). Mann-Whitney U tests were used for comparisons of continuous variables between the studied groups. A p value of <0.05 was considered significant.
RESULTS
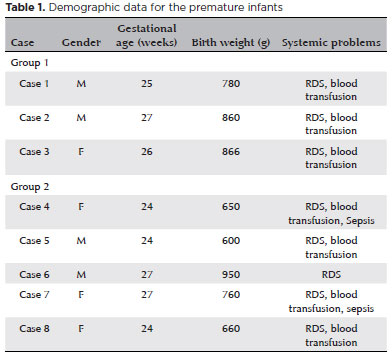
IVB was administered to the 15 eyes of eight patients (four girls, four boys) as the first treatment. A flat demarcation line and plus disease or stage-3 disease was present at posterior zone 2 in all eyes. Group 1 consisted of two boys and one girl, and Group 2 of two boys and three girls. Mean gestational ages and mean birth weights are shown in table 1. There were no significant differences in ages or weights between the two groups (p>0.05). Retinal vascularization was completed without any additional treatment in the five eyes in Group 1. The mean completion time of retinal vascularization was postmenstrual 53.6 ± 1.5 (52-55) weeks. Adjuvant LPC was performed due to relapse in five of the 10 eyes in Group 2. The mean LPC administration time was postmenstrual 40.6 ± 4.5 weeks. The mean number of laser spots used in the five eyes was 1.500.6 ± 152.38 spots. Retinal vascularization was completed in postmenstrual 47.6 ± 1.5 (46-49) weeks in the remaining five eyes. The mean follow-up duration for all cases was 61.5 ± 17.1 (46-94) weeks.
A fibrovascular membrane extending into the vitreous at the ridge area for approximately 4 hours developed in one eye after LPC (Case 5, left eye) and regressed without retinal traction during follow-up. A second demarcation line developed at zone 3 over the area with laser spots in two eyes (Case 7, bilaterally), and this demarcation line later regressed. None of the patients developed complications, such as cataract, glaucoma, retinal tear, retinal or vitreous hemorrhage, or retinal detachment.
Although respiratory distress syndrome (RDS) was present in all cases in the study, blood transfusion was required in seven cases (except Case 6) and sepsis developed in two cases (Case 4: Candida; Case 7: Pseudomonas aeruginosa) (Table 2). Retinal vessels had passed the demarcation line at the time of initial diagnosis in the eyes requiring adjuvant LPC, and a second ROP line was found at the posterior border of zone 2, enabling treatment with fewer laser spots. We did not observe any changes in blood pressure or any systemic problems that could have been systemic side effects in any patients following treatment.
DISCUSSION
Aggressive posterior ROP is a rare disease and constitutes 10% of the ROPs requiring treatment(7). Progression can occur in eyes with AP-ROP despite conventional treatment, leading to unfavorable developments that may include retinal detachment, macular dragging, and macular fold in approximately 55%-78% of cases in controlled, prospective studies(7). Anti-VEGFs, and especially bevacizumab, have become commonly used following these disappointing results, especially for the treatment of AP-ROP, in various clinics after the first use of antiVEGFs in posterior ROP treatment in 2007(8,9). Anti-VEGFs are used in ROP treatment as monotherapy or are combined with either LPC or vitrectomy(7,8,10-13). Although some authors recommend monotherapy with anti-VEGFs to allow the development of retinal vessels along the demarcation line, others, such as Kim et al.(14), suggest that bevacizumab in addition to laser treatment is more effective in type-1 disease in zone 1(12). However, one must be careful when using anti-VEGFs in stage-4 and stage-5 ROP as they may accelerate the retinal detachment by increasing membrane contraction(15,16).
Only small amounts of bevacizumab pass into the systemic circulation when the retina is intact(17). Moreover, the half-life of bevacizumab in the vitreous of preterm infants, which is quite viscous, is relatively long (5-10 days) and a single-dose IVB administration can be sufficient for ROP treatment(17-19). There is no consensus on the optimal dose of bevacizumab to be used in newborns for ROP treatment(20). Mintz-Hittner et al.(6) recommended 0.625 mg of bevacizumab, which is half the adult dose, in the BEAT-ROP study. Although successful regression of ROP has been reported with various doses from 0.16 to 1.25 mg, the generally used dose is 0.625 mg, as recommended in the BEAT-ROP study(21-23). However, using the 4/3 × p × r3 formula with the axial length of the eye and the vitreous length in newborns, as compared with adult values, reveals the newborn eye to be one third the size of the adult eye. The size-adjusted dose in newborns should therefore be 0.4 mg(24). Furthermore, although the drug levels for all VEGFs to be bound should be 2.6/1, the necessary ratio to prevent endothelial cell migration has been shown to be 10/1 in in vitro studies(25). These results indicate that the current bevacizumab doses used in ROP treatment are much higher than required for VEGF blockage. Harder et al.(12) administered 0.375 mg/0.03 ml as a single dose of IVB to 23 eyes of 12 patients and reported 100% regression. Connor et al.(22) administered 0.16 mg of IVB to one eye and 0.32 mg of IVB to the other eye for a patient with AP-ROP and observed regression in both eyes. However, the disadvantage of very low doses is the requirement for the drug to be diluted for administration. Some articles report that ranibizumab and bevacizumab have a similar effect, while others report more reactivation following ranibizumab(26-28). Aflibercept was also shown to be effective in high-risk type-1 prethreshold ROP in a study conducted by Salman et al.(29). We administered 0.5 and 0.625 mg of bevacizumab. Though AP-ROP regressed following the administration of both doses, relapse was more common with 0.5 mg of bevacizumab, with LPC being required in half of these eyes.
The current information on the long-term ocular and systemic side effects of these drugs is inadequate and there are several concerns. VEGF expression has been shown in the lungs, kidneys, and brains of newborns(30). Bevacizumab administered at doses of 0.5 mg and 1.0 mg of in premature babies in whom LPC was administered was shown to pass to the systemic circulation and decrease serum VEGF levels in a study conducted by Sato et al.(21). LPC, as well as the increased vascular permeability in ROP, may promote the passage of bevacizumab into the systemic circulation. However, our knowledge on the pharmacokinetics of these drugs in the vitreous or serum of premature infants, where maturation of the tissues is incomplete, is limited(30). Although bevacizumab administration in premature infants reportedly did not increase mortality in the BEAT-ROP study, the long-term effects of systemic VEGF inhibition on the development of organs are not known(6). Another randomized controlled study (clinical trial identifier: NCT01232777) titled "Pan-VEGF blockade for the treatment of ROP (BLOCK-ROP)" investigating the effectiveness and reliability of IVB and comparing 0.625 and 0.75 mg of bevacizumab with photocoagulation is still ongoing. We did not observe any local or systemic side effect changes in any patient following treatment.
The limitations of this study were its retrospective design, the small number of patients, and the fact that the criteria used for relapse were less definitive than the criteria used for primary ROP. There are many questions to be answered concerning the use of anti-VEGF in different doses. The relevant doses mentioned in the literature are very small and difficult to adjust during administration. Therefore, it would not be surprising to administer doses that are different than planned, especially when very small doses are administered following dilution. Our main aim in this retrospective study was to determine whether there should be a preference for very low or high doses of bevacizumab in AP-ROP treatment. However, we did not aim to discuss the long-term results of anti-VEGF administration in premature babies. Retinal vascularization completion took longer in Group 1, which received a higher dose compared to Group 2; however, some Group 2 patients required additional treatment. We believe lower doses can increase the need for additional treatment, while larger doses can led to the need for increased monitoring time. Therefore, we feel our findings should be discussed based on these observations, despite the inability to conduct statistical analyses due to the small number of patients. Such a small patient population is an important limitation of this study, but we feel anecdotal information is also important when premature babies are concerned. We also hope our data will attract other investigators to this field. Larger studies with long-term outcomes are required to determine the effectiveness and safety of bevacizumab or anti-VEGFs at various doses.
REFERENCES
1. Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1993;3(1):26-32.
2. Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77-82.
3. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophtalmol. 2005;123(7):991-9.
4. Cryotherapy for Retinopathy of Prematurity Cooperative Group. The natural ocular outcome of premature birth and retinopathy. Status at 1 year. Arch Ophthalmol. 1994;112(7):903-12.
5. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684-94.
6. Mintz-Hittner HA, Kennedy KA, Chuang AZ, for the BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364(7):603-15.
7. Wutthiworawong B, Thitiratsanont U, Saovaprut C, Subhanqkasen I, Geyuraphun B, Ampornprut A, et al. Combined intravitreal bevacizumab injection with laser treatment for aggressive posterior retinopathy of prematurity (AP-ROP). J Med Assoc Thai. 2011;94 Suppl 3:S15-21.
8. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone 1 retinopathy of prematurity. Grafes Arch Clin Exp Ophthalmol. 2007;245(11):1727-30.
9. Travassos A, Teixeria S, Ferreira P, Regadas I, Travassos AS, Esperancinha FE, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007;38(3):233-7.
10. Arambulo O, Dib G, Iturralde J, Duran F, Brito M, Fortes Filho JB. Intravitreal ranibizumab as a primary or a combined treatment for severe retinopathy of prematurity. Clinical Ophthalmology 2015; 9:2027-32.
11. Cernichiaro-Espinosa LA, Olguin-Manriquez FJ, Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, Martinez-Castellanos MA. New insights in diagnosis and treatment for Retinopathy of Prematurity. Int Ophthalmol. 2016; 36(5):751-60.
12. Harder BC, Baltz SV, Jonas JB, Schlichtenbrede FC. Intravitreal bevacizumab for retinopathy of prematurity. J Ocul Pharmacol Ther. 2011;27(6):623-7.
13. Autrata R, Krejcírová I, Senková K, Holousová M, Dolezel Z, Borek I. Intravitreal pegaptanib combined with diode laser therapy for stage 3+ retinopathy of prematurity in zone I and posterior zone II. Eur J Ophthalmol. 2012;22(5):687-94.
14. Kim J, Kim SJ, Chang YS, Park WS. Combined intravitreal bevacizumab injection and zone 1 sparing laser photocoagulation in patients with zone 1 retinopathy of prematurity. Retina. 2014; 34(1):77-82.
15. Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the membrane after intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Grafes Arch Clin Exp Ophthalmol. 2008;246(7):1061-3.
16. Zepeda-Romero LC, Liera-Garcia JA, Gutiérrez-Padilla JA, Valtierra-Santiago JI, Cardenas-Lamas LJ. Paradoxical vascular-fibrotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye (Lond). 2010;24(5);931-3.
17. Mintz-Hittner HA. Avastin as a monotherapy for retinopathy of prematurity. J AAPOS. 2010;14(1):2-3.
18. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114(5):855-9.
19. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008;146(4):508-12.
20. Spandau U, Tomic Z, Ewald U, Larsson E, Akerblom H, Holmström G. Time to consider a new treatment protocol for aggressive posterior retinopathy of prematurity? Acta Ophthalmol. 2013;91(2):170-5.
21. Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, et al. Serum Concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153(2):327-33.
22. Connor AJ, Papastavrou VT, Hillier RJ, Shafiq A. Ultra-low dose of intravitreal bevacizumab in the treatment of retinopathy of prematurity. J Pediatric Ophthalmol Strabismus. 2015; 52:e20-1.
23. Micieli JA, Surkont M, Smith AF. A systematic analysis of the off-label use of bevacizumab for severe retinopathy of prematurity. Am J Ophthalmol. 2009; 148(4):536-543.e2.
24. Spandau U. What is the optimal dosage for intravitreal bevacizumab for retinopathy of prematurity? Acta Ophthalmol. 2013; 91(2):e154.
25. Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7(4):335-45.
26. Erol MK, Çoban DT, Sari ES, Bilgin AB, Dogan B, Özdemir Ö, et al. Comparison of intravitreal ranibizumab and bevacizumab treatment for retinopathy of prematurity. Arq Bras Oftalmol. 2015;78:340-3.
27. Wong RK, Hubschman S, Tsui I. Reactivation retinopathy of prematurity after ranibizumab treatment. Retina. 2015;35(4):675-80.
28. Chen SN, Lian I, Hwang YC, Chen YH, Chang YC, Lee KH, et al. Intravitreal antivascular endothelial growth factor treatment for retinopathy of prematurity: comparison between Ranibizumab and Bevacizumab. Retina. 2015;35(4):667-74.
29. Salman AG, Said AM. Structural, visual and refractive outcomes of intravitreal aflibercept injection in high-risk prethreshold type 1 retinopathy of prematurity. Ophthalmic Res. 2015;53(1):15-20.
30. Hård AL, Hellström A. On safety, pharmacokinetics and dosage of bevacizumab in ROP treatment: a review. Acta Paediatr. 2011; 100(12):1523-7.
Submitted for publication:
May 24, 2017.
Accepted for publication:
August 8, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Approved by the following research ethics committee: İnönü University Turgut Özal Medical Center (# 2016/9-4).