INTRODUCTION
Retinal vein occlusion (RVO) is the second most common vascular condition capable of causing irreversible vision loss1. Reduced visual acuity (VA) in patients with RVO is usually because of macular edema (ME) and/or locally impaired capillary perfusion2-7. Both the location and total area of capillary non-perfusion greatly affect the duration of the disease and risk of proliferative complications6-10. Post-occlusion ME is typically caused by local inflammation and the effects of increased Vascular Endothelial Growth Factor (VEGF) levels2,6-10. VEGF expression is potentiated by tissue pH and the partial pressure and concentration of oxygen; hence, hypoxia induces VEGF synthesis in ischemic RVO2.
Previously, the relative incidences of ischemic versus non-is chemic RVO were reported to be 19% and 81%, respectively11. Since the implementation of wide-field fluorescein angiography (FA) in clinical practice, it has been established that 60%-80% of patients with RVO have large areas of capillary non-perfusion in the mid- and far-peripheral retina, occupying 41-415 mm2 (23-348 optic disc areas)12,13.
In most of these patients, foveal function is preserved, which explains the rapid improvement in their VA if treated with inhibitors of angiogenesis. However, when prescribing ranibizumab for RVO-related ME, certain limitations specified in its Summary of Product Characteristics (SPC) must be considered. The SPC for ranibizumab states that there are limited data on its use to treat patients with prior episodes of RVO or patients with ischemic branch RVO or central RVO (CRVO), and that its administration is not recommended in patients with RVO presenting clinical signs of irreversible ischemic loss of visual function14. In other words, RVO-associated ischemic maculopathy is a contraindication for the use of ranibizumab. However, whether this is true for peripheral ischemic retinopathy remains unknown. A recent study suggested that prolonged use of anti-VEGF agents decreases the rate of progression of retinal ischemia and reduces neovascularization (without reducing the risk of its occurrence) in patients with CRVO at high risk of iris neovascularization15. In our opinion, the optimal treatment for patients with peripheral ischemia and RVO is ranibizumab therapy combined with scatter laser photocoagulation (SLP) of non-perfused areas of the retina. We suggest that this combination shortens the treatment period and lowers the risk of neovascular complications, both of which reduce the cost of rehabilitation12,16.
Panretinal laser photocoagulation is the gold-standard therapy for ischemic CRVO complicated by iris and/or angle neovasculariza tion1. However, it does not improve vision or resolve ME. CRVO in vestigators propose the exclusive use of classical panretinal laser pho tocoagulation (including for early and preventive treatment), whereas we only use selective SLP of non-perfused areas1.
Anti-VEGFs are also used to treat post-CRVO ME, although they have no beneficial effect on ischemia and fail to prevent neovascularization in some patients. In this study, we sought to identify a combined therapy that caused as little laser injury as possible to improve VA without adverse effects.
METHODS
In this prospective study, we enrolled 250 patients with CRVO (135 women, 115 men; mean age, 62.4 ± 12.5 years) treated at the Ophthalmology Department of the First Pavlov State Medical Uni versity of St. Petersburg, St. Petersburg, Russia, between 2010 and 2014. The mean interval between disease onset and treatment initiation was 1.5 ± 1.2 months, and the average follow-up period was 24.5 ± 6.5 months. Patients were eligible for inclusion if they had: experienced an episode of CRVO in the preceding 6 months; a VA of ≥0.02 on the Snellen chart; and a central retinal thickness (CRT) of ≥450 µm. Patients were excluded if they: were aged under 18 years; were pregnant; had suffered uncontrolled arterial hypertension, stroke, or myocardial infarction in the preceding 12 months; or had ocular inflammation, media opacity, or unstable primary glaucoma.
Retinal optical coherence tomography was performed at the first visit and repeated monthly during the follow-up period (SPECTRALIS® OCT; Heidelberg Engineering GmbH, Heidelberg, Germany). FA was performed at the first appointment in all patients and repeated at Months 3, 6, 12, and 24-26 in patients with retinal ischemia (Hei delberg Retina Angiograph 2; Heidelberg Engineering). We measured the non-perfused area in every angiographic image of the nine to 13 an giograms necessary to observe most of the retina with a 55º lens (therefore, the term multi-field FA is appropriate) and calculated the total non-perfused area (Figure 1). Two FA specialists separately outlined the non-perfused areas. Areas of non-perfusion were mea sured in mm2 in unprocessed angiograms using pre-installed Heyex software v.1.7.0.0 (Heidelberg Engineering). We did not perform any manual adjustments to calculate ischemic indices in contrast to other researchers17.

Figure 1 (A) and (B) Areas of capillary non-perfusion outlined in nine standard angiographic images from two different patients. To avoid overstating the total area involved because of the overlap of neighboring images, anatomic structures (such as blood vessels) were used as landmarks.
Data analyses were performed using the Statistica 7 software suite (StatSoft, Tulsa, OK, USA) and included the estimation of х ± δ values and their dispersion and covariation coefficients at different stages of the study. Differences were considered significant at p<0.05.
After examination, patients were divided into three groups based on CRVO type: non-ischemic (those who received ranibizumab treat ment and were not included in further comparisons and analyses); and two comparable (Table 1) ischemic groups that received different treatments. Patients with 10 disc areas or more of retinal capillary non-perfusion were considered to have ischemic CRVO.
Table 1 Baseline characteristics of the patients and data on the study outcome
| Characteristic | Ranibizumab and SLP group (#1) n=88 | Ranibizumab alone group (#2) n=87 | p value |
|---|---|---|---|
| Baseline | |||
| Mean age, years | 062.50 ± 012.90 | 061.70 ± 011.40 | <0.38 |
| Female sex, n (%) | 52 (59.1) | 53 (60.1) | <0.41 |
| Disease duration, months | 1.40 ± 1.10 | 01.60 ± 1.30 | <0.34 |
| Retinal ischemia area, mm2 | 410.20 ± 157.25 | 425.50 ± 210.14 | <0.28 |
| BCVA, Snellen chart | 0.25 ± 0.15 | 00.27 ± 0.09 | <0.26 |
| CRT, µm | 524.02 ± 243.85 | 535.04 ± 210.12 | <0.32 |
| Outcome | |||
| BCVA by month 28, Snellen chart | 0.41 ± 0.25 | 00.40 ± 0.15 | <0.29 |
| CRT by Month 19*, µm | 270.52 ± 123.81 | 287.04 ± 130.44 | <0.19 |
| Mean number of ranibizumab injections | 3.50 ± 1.60 | 10.60 ± 2.50 | <0.01 |
Optical coherence tomography was performed in only a few patients between 19 and 28 months; therefore, we did not include these data in the analysis. BCVA= best corrected visual acuity (assessed using a Snellen chart); CRT= central retinal thickness assessed by optical coherence tomography; SLP= scatter laser photocoagulation.
Non-ischemic patients with CRVO received intravitreal injections of 0.5 mg ranibizumab scheduled as recommended by the manufacturer (Lucentis®; Novartis Pharma AG, Basel, Switzerland). This group was only examined to determine the prevalence of ischemia among patients with CRVO according to wide-field angiography results, and was not included in further analyses.
Group 1 received combined treatment: intravitreal injections of 0.5 mg ranibizumab every month for a 3-month period and selective peripheral SLP of non-perfused areas of the retina. SLP sessions were performed 30 min before the first intravitreal injection in all patients and repeated at months 1 and 2 as needed. The following settings were applied: laser wavelength, 514 nm; spot diameter, 400 µm; exposition, 0.15 s, and an energy level sufficient to produce a white coagulate. In some patients, we were unable to perform selective peripheral SLP of all the non-perfused areas in one session (for example, if the non-perfused area was too large and the patient experienced too much pain, or if we had to wait until hemorrhages had resolved in the retina). Laser treatment was then repeated at months 1 and 2, according to the opinion of the laser and FA specialists. It should be noted that this approach can leave a considerably large non-perfused area untreated for a relatively long time, which can interfere with VA and CRT. However, the main reason for "deferred" SLP was the presence of retinal hemorrhages, and it was impossible to apply complete SLP before they had resolved (a process that takes up to 3 months). If all non-perfused areas were coagulated in one SLP procedure, SLP was not repeated unless the non-perfused areas were found to have increased at follow-up FA.
Following the administration of the first three monthly intra vi treal injections, which was after the patients had completed laser treatment, ranibizumab therapy was continued pro re nata (PRN) for another 24 months. An additional injection was considered necessary in patients who demonstrated retinal thickening of ≥150 µm and related significant vision loss. We defined a "significant" VA decrease as a loss of at least two lines on the Snellen chart in patients whose maximum achieved best corrected visual acuity (BCVA) was ≥0.4, and a loss to 0.05 in those whose maximum BCVA was 0.1 on the Snellen chart. In patients with a VA >0.1 and <0.4, reinjection was performed if there was a loss of one line on the Snellen chart.
Patients in Group 2 received monthly ranibizumab monotherapy for the first 3 months and switched to a PRN regimen for the following 24 months.
These treatment regimens and reinjection criteria were selected for economic reasons. In Russia, patients with RVO receive no reimbursement for Lucentis® treatment (and use of intravitreal Avastin® [F. Hoffmann-La Roche Ltd, Basel, Switzerland] is prohibited). Therefore, many prefer to wait as long as possible for treatment based on their individual threshold. Thus, patients requested therapy when their vision was very poor. The rationale for the three monthly doses at the start of treatment was that we continued fixed monthly dosing until the improvement in VA was stable on two consecutive visits (in many patients, this occurred at months 2 and 3 or 3 and 4) and started PRN treatment after that. The mean number of injections administered with a fixed regimen was three.
The eye function and edema of patients in this study were worse than those of patients in the CRUISE study, in which no patients with ischemic RVO were included18. The conventional criteria of a VA <0.5 and CRT ≥250 µm were therefore not applicable. According to these criteria, we should have used fixed dosing for a longer period, which our patients could not afford.
RESULTS
Multi-field angiography revealed capillary non-perfusion in 175/250 patients with CRVO (70%). Thus, Group 1 comprised 88 pa tients and Group 2 comprised 87 patients. The mean total area of retinal ischemia was 435.12 mm2 (standard deviation [SD], ± 225.13 mm2), i.e., 167.15 optic disc areas (SD, ± 45.16). We diagnosed peripheral ischemia in 125 patients, corresponding to 50% of the total number of patients with CRVO and 71.4% of patients with any type of ischemia. The mean total area of peripheral retinal ischemia was 370 mm2 (SD, ± 113.5 mm2), i.e., 142.21 (SD, ± 85.12) optic disc areas.
In Group 1, the mean VA at baseline was 0.25 ± 0.15, and the mean central macular thickness was 524.02 ± 243.85 µm. In the PRN treatment period (24 months), patients in this Group received on average 3.5 ± 1.6 intravitreal ranibizumab injections. This means that a large proportion of patients in this Group received only the loading dose in the 2-year follow-up period. The average number of laser burns was 1320 ± 245. After the third injection, VA usually reached its maximum (0.52 ± 0.15), and the central macular thickness decreased to 270.7 ± 151.34 µm. In a month, these parameters had decreased in all patients, reaching 0.41 ± 0.25 µm and 270.5 ± 123.8 µm, respectively, by the end of treatment. There were, however, 12 patients in whom the area of impaired capillary perfusion increa sed by 25-60 mm2 over the total observation period, necessitating additional laser treatment. There was no correlation between the progression of ischemia and the number of injections.
The average number of injections administered after peripheral SLP sessions was 2.9 ± 1.4. There was no correlation between final VA or final CRT and the total number of injections. As expected, a po sitive statistical relationship was found between the area of ischemia and the number of laser burns, as well as between the area of ischemia and CRT (both initial and final). Importantly, by the end of the study, this Group had reduced in number by three patients. One of them received an insufficient number of peripheral SLP sessions, and presented at Month 15 with neovascular glaucoma and eye pain, necessitating diode laser transscleral cyclophotocoagulation and panretinal photocoagulation (PRP); data from the other two patients were unavailable.
Patients in Group 2 received an average of 10.6 ± 2.5 intravitreal injections during the 24-month period. In this group, the mean VA at baseline was 0.22 ± 0.2 (Snellen chart), and the mean central macular thickness was 626.13 ± 298.06 µm. The highest VA and lowest retinal thickness, 0.45 ± 0.21 and 290.7 ± 214.5 µm, respectively, were registered after the third injection.
By the end of the study, 15 patients demonstrated a 45.5 ± 15.6 mm2 increase in the area of ischemia. Iris neovascularization occurred in five patients an average of 2.5 months after the completion of ra nibizumab therapy, necessitating PRP (in two patients, diode laser transscleral cyclophotocoagulation was also performed). Two other patients developed posterior segment neovascularization and underwent additional treatment with PRP. Anterior segment neovas cularization occurred in only one patient in Group 1. All other ins tances of neovascularization occurred in patients treated with ra nibizumab alone (Group 2).
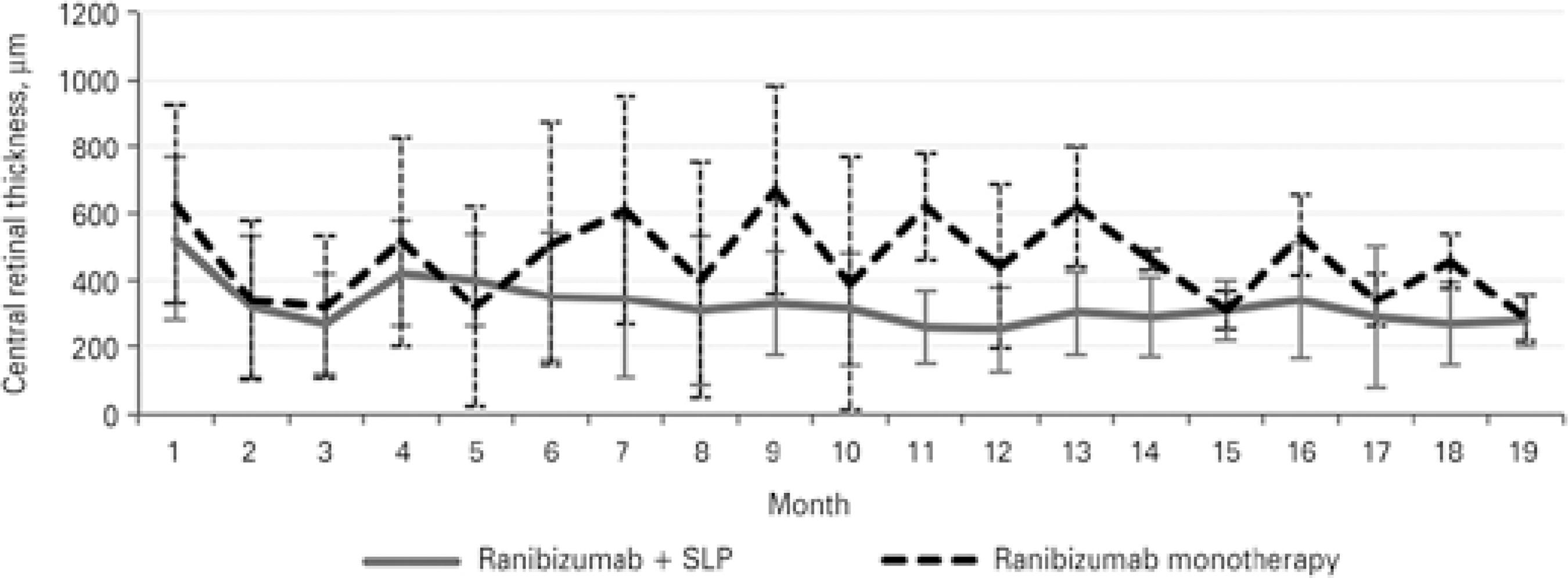
The dynamics of changes in VA differed between groups 1 and 2. In Group 1, VA was lowest at the 5-month follow-up and then remained generally stable, showing only minor fluctuations. In Group 2, VA showed no stabilization, and its fluctuations were large (Figure 2). CRT changes correlated well with VA changes (Figure 3). In both ischemic CRVO groups, the initial and final VA, initial and final CRT, and number of anti-VEGF injections were positively correlated with the area of capillary non-perfusion.
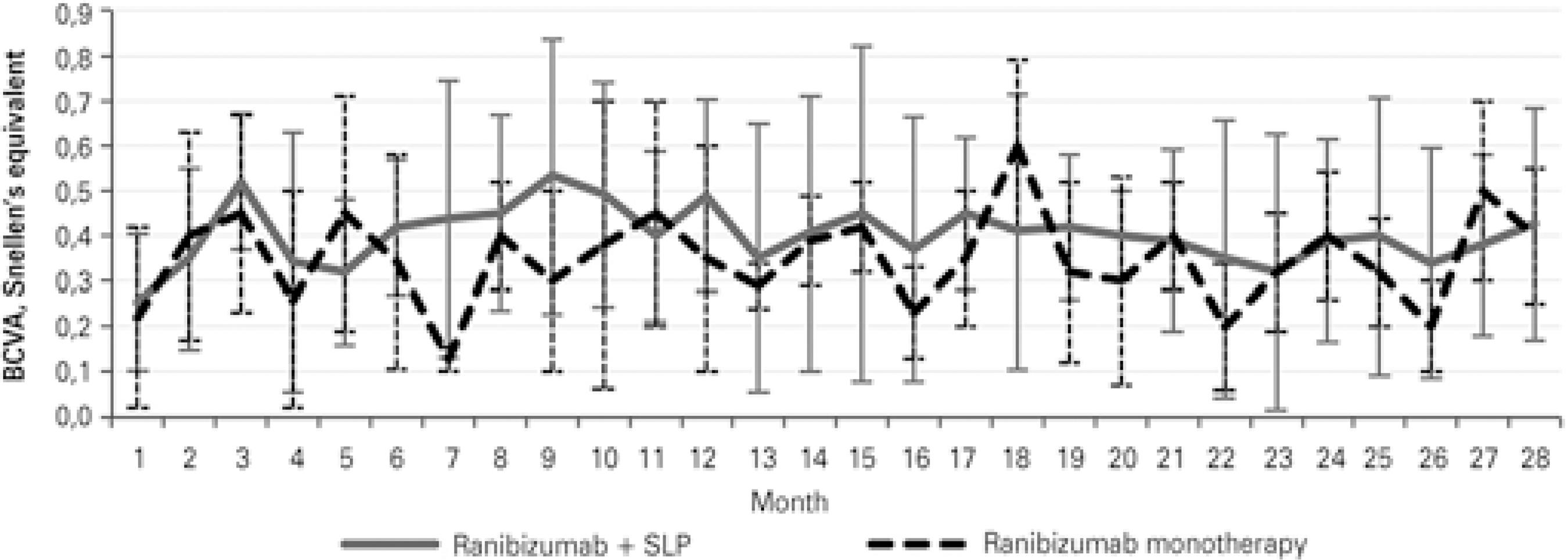
Figure 2 Mean visual acuity changes with 95% confidence intervals in groups receiving different therapies.
DISCUSSION
The use of multi-field angiography for the primary assessment of patients with CRVO enables the detection of retinal ischemia in almost 70% of patients. In most patients, ischemic changes are loca ted at the periphery of the retina. Their total area is usually large, occupying on average 370 mm2 (SD ± 113.5 mm2), i.e., 142.21 (SD, ± 85.12) optic disc areas. Patients who fall into this category are at higher risk of proliferative complications; therefore, specific ischemic RVO management algorithms should be followed.
Notably, foveal function is usually preserved in these patients, ensuring a response to anti-VEGF agents. Ranibizumab monothe rapy, however, provides only temporary effects. After injections stop, patients' vision deteriorates again, and ME recurs. For a more stable result, further injections are required. However, further injections confer a higher risk of complications and increased medical costs, making this treatment unfeasible for our patients. In our study, even prolonged (24-month) anti-VEGF therapy failed to reduce the risk of neovascularization. However, we found no correlation between the number of ranibizumab injections and progression of ischemia in any of our patients. When combined with selective SLP of non-perfused areas of the retina, invasive intravitreal therapy can be minimized. The advantages of this approach include reduced medical costs, a shorter treatment period, and faster stabilization.
In a small randomized study, laser photocoagulation of peripheral areas of non-perfusion did not decrease injection frequency or improve VA in eyes with CRVO treated with ranibizumab19. The authors proposed that the complete loss of vascularity of the peripheral retina caused neuroretinal infarction and consequently no long-term VEGF production. Such patients may require less aggressi ve anti-VEGF treatment. However, there are many publications on the positive correlation between the area of non-perfusion and intraocular VEGF levels, and in our opinion it is essential to treat all patients with an ischemic retina.
Regarding patients in whom the non-perfused area showed progression over time, we consider the persistently reduced perfu sion pressure between the arterial and venous vascular retinal network to be one of the main causative factors. Impaired Endothelin-1-induced autoregulation and neurovascular coupling may contribute to this process, with retinal venous pressure changes accompanying CRVO19,20. Further studies involving more patients and the measurement of perfusion pressure are needed to provide an answer to this question.
The main strengths of this pilot study are the number of patients observed and long follow-up period. The limitations of this study include the absence of randomization, probable ranibizumab undertreatment for local economic reasons, relative inaccuracy of BCVA measurements using the Snellen chart, and the possible "deferred laser" effect discussed in the Methods section. More experience in patients with peripheral ischemic CRVO over a longer follow-up period is needed.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

