INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide(1,2). This disease is associated with chronic and progressive optic neuropathy(1,3) characterized by loss of retinal ganglion cells(3), which leads to visual field deterioration(1,3,4). Moreover, glaucoma is associated with traffic accidents, restricted mobility, and falls, thus affecting qua lity of life(1). An important risk factor for glaucoma is intraocular pressure (IOP), and its decrease is the mainstay of treatment(3).
IOP measurement has been a matter of debate for many years. In 1950, Goldmann introduced an approach to measure IOP, which is called applanation tonometry, and this approach is currently the gold standard(3-5). This approach is related to the elasticity of the cornea, which indicates that it depends on corneal thickness and hysteresis(4). Goldmann assumed that the average central corneal thickness (CCT) would be approximately 500 µm(4-7), and excessively thin and thick corneas would cause underestimations and overestimations of the IOP, respectively(4,7,8). With the advent of more sophisticated devices capable of measuring CCT, it has become clear that CCT is much more variable than predicted by Goldmann(5-7). More recently, some studies, such as the Ocular Hypertension Treatment Study (OHTS), stated that CCT is an important confounder of Goldmann applanation tonometer (GAT) measurements(5,6,8). In addition, factors, such as astigmatism, examiner's competence, gaze direction, tear thickness, corneal hydration, connective tis sue composition, bioelasticity, corneal curvature, and other corneal biomechanical properties, are important sources of error in GAT measurements(2-4,8). Currently, an accepted formula to correct IOP is not available(4,6,7).
The ocular response analyzer (ORA) was introduced in 2005, and it was classified as a non-contact tonometer(2,3,5,9). This tonometer allows the measurement and evaluation of corneal biomechanical properties, namely corneal hysteresis (CH), corneal resistance factor (CRF), and corneal compensated intraocular pressure (IOPcc)(3,5), as well as CCT and Goldmann correlated intraocular pressure (IOPg)(3,5). Briefly, the ORA produces a rapid air pulse that deforms the corneal curvature(2,3,5,9) and records corneal deformation(2,9). When the cornea moves inward, it reaches the first applanation state (P1)(2,3,9). After a slightly concave state(2,3,9), the air pulse pressure decreases and the cornea moves outward, passing through the second applanation state (P2)(2,3,9). The average of P1 and P2 is IOPg, which is analogous to the IOP measured by GAT(2,5,9), being the difference between these two values (P2 - P1) the value of CH(2,3,5).
The OHTS revealed that CCT is an important and independent risk factor for the development of glaucoma(4-6,10). These results were validated in the European Glaucoma Prevention Study (EGPS)(4,5). In fact, a two-fold increased risk of progression to glaucoma over 5 years was found for each 40-mm thinning of the central cornea(4), indicating that a patient with a thin cornea has a high risk of glaucoma progression(4,6). However, this was not noted in other studies. For instance, in the Early Manifest Glaucoma Trial (EMGT), with 5 years of follow-up, CCT was not a significant predictive factor for glaucoma progression(4). The value of CCT as a significant predictive factor for the progression of glaucoma was only noted in patients with high baseline IOP and not in those with low baseline IOP after 11 years of follow-up(4). Furthermore, other studies such as the Barbados Eye Study and the studies by Chauhan et al. and Congdon et al., did not find any association between CCT and glaucoma(2,4).
Interestingly, Congdon et al. showed that CH is associated with glaucoma progression risk(2,5,9). This finding suggests that low CH is associated with glaucomatous visual field damage and optic nerve defects(2,9). In fact, CH may be more strongly associated with glaucoma diagnosis, risk of progression, and effectiveness of glaucoma treatment than CCT itself(2,9).
Nevertheless, the biological link between the biomechanical pro perties of the eye and glaucoma development and progression remains to be understood(4-6).
The present study aimed to investigate the associations of CH and CCT with glaucoma development.
METHODS
Our study is the first review of the literature and meta-analysis to collect CCT and CH data from adults with glaucoma and heathy controls in order to discuss differences in these two outcomes in both groups. This study was conducted in July 2016.
Eligibility criteria
In this study, we only considered observational studies that included adult patients with a diagnosis of open-angle glaucoma and controls and that reported CCT and CH as outcomes.
Studies with any other ophthalmologic diagnosis that could affect IOP, those not written in English, those with an interventional design, those with a non-healthy control group, those with pediatric patients and volunteers (age <18 years), and those that did not provide outcome values for each group separately were excluded.
In order to minimize confounding factors, only studies with primary open-angle glaucoma (POAG) were analyzed. Moreover, only non-interventional studies were considered to reduce the possible interference of procedures and medications with the pri mary outcome.
Information sources and search procedure
MEDLINE was used as the information source, and the search terms used were "hysteresis," "glaucoma," and "corneal thickness" between January 2006 and July 2016. This limited search period was because of the introduction of the ORA, which allows the measurement of CH(3), only in 2005(2,9). As CH and CCT were our primary outcomes, we used the above-mentioned search terms in order to obtain access to a non-restrictive group of studies on this topic for further consideration.
Study selection
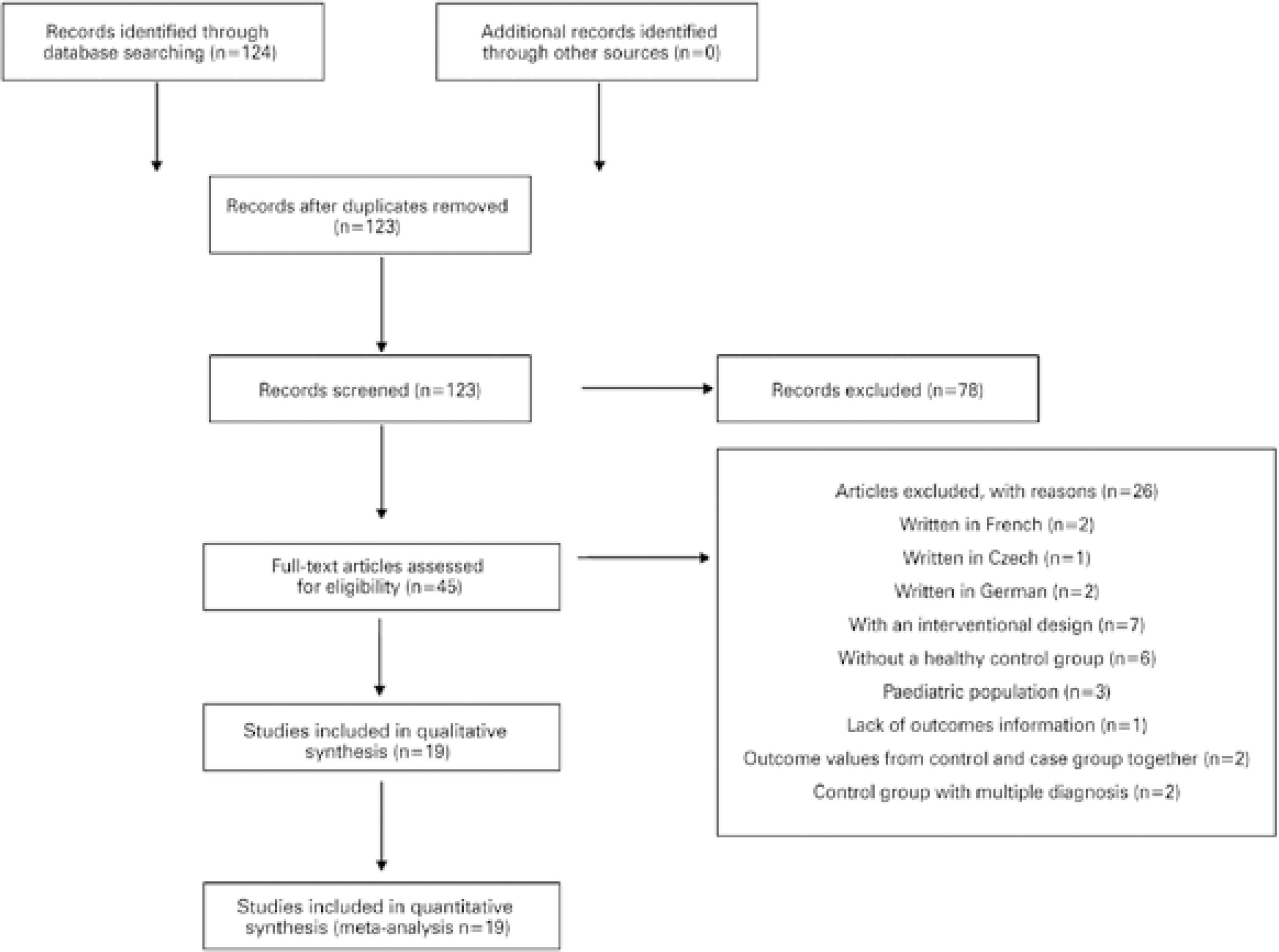
A total of 124 articles were identified with the search criteria. The abstract from each article was used for screening, and one of the abstracts was found to be duplicated. After screening, we found 45 studies, and of these, 2 were written in French, 2 were written in German, 1 was written in Czech, 3 included pediatric populations, 1 had no outcome information, 2 had a case group with a glaucoma diagnosis and other diagnoses, 2 provided data from the control and case groups together, 6 had a non-healthy control group, and 7 were interventional studies.
Therefore, for comparative and quantitative purposes, 19 studies between 2008 and 2016 were considered (Table 1)(11-29). The study selection information is presented in figure 1, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines(30).
Table 1 Baseline characteristics
| Article | Diagnosis | Nº of patients | Nº of eyes | CH | CCT |
|---|---|---|---|---|---|
| Mean ± SD (mmHg) | Mean ± SD (µm) | ||||
| Kurysheva, 2016(11) | Glaucoma | 32 | 32 | 10.10 ± 1.60 | 548.10 ± 31.30 |
| Control | 30 | 30 | 11.20 ± 1.70 | 549.30 ± 30.80 | |
| Pillunat, 2016(12) | Glaucoma | 48 | 48 | 8.54 ± 1.86 | 530.60 ± 38.40 |
| Control | 44 | 44 | 10.49 ± 1.67 | 556.20 ± 37.00 | |
| Shin, 2015(13) | Glaucoma | 97 | 97 | 9.90 ± 1.66 | 548.30 ± 34.82 |
| Control | 89 | 89 | 10.59 ± 1.71 | 558.77 ± 31.19 | |
| Yazgan, 2014(14) | Glaucoma | 30 | 30 | 6.80 ± 1.70 | 509.00 ± 36.00 |
| Control | 45 | 45 | 10.30 ± 1.50 | 546.30 ± 28.00 | |
| Costin, 2014(15) | Glaucoma | 13 | 13 | 9.02 ± 1.52 | 546.70 ± 35.00 |
| Control | 15 | 15 | 10.26 ± 1.30 | 546.10 ± 35.50 | |
| Insull, 2010(16) | Glaucoma | 38 | 38 | 8.80 ± 1.52 | 532.00 ± 33.46 |
| Control | 62 | 62 | 9.60 ± 1.49 | 550.00 ± 35.43 | |
| Sullivan-Mee, 2012(17) | Glaucoma | 116 | 116 | 7.76 ± 1.60 | 541.00 ± 36.00 |
| Control | 67 | 67 | 9.54 ± 1.60 | 552.00 ± 35.00 | |
| Kaushik, 2012(18) | Glaucoma | 36 | 36 | 7.90 ± 2.80 | 523.50 ± 35.50 |
| Control | 71 | 71 | 9.50 ± 1.40 | 530.70 ± 33.40 | |
| Detry-Morel, 2012(19) | Glaucoma | 30 | 30 | 9.20 ± 1.10 | 544.00 ± 37.00 |
| Control | 25 | 25 | 10.80 ± 1.60 | 554.00 ± 19 .00 | |
| Morita, 2012(20) | Glaucoma | 83 | 83 | 9.20 ± 1.30 | 535.40 ± 24.90 |
| Control | 83 | 83 | 10.80 ± 1.30 | 541.40 ± 26.80 | |
| Cankaya, 2011(21) | Glaucoma | 78 | 78 | 6.90 ± 2.10 | 537.90 ± 35.20 |
| Control | 102 | 102 | 9.40 ± 1.40 | 539.80 ± 25.90 | |
| Grise-Dulac, 2012(22) | Glaucoma | 38 | 75 | 10.03 ± 2.31 | 551.50 ± 38.90 |
| Control | 22 | 44 | 11.05 ± 1.53 | 550.70 ± 29.30 | |
| Detry-Morel, 2011(23) | Glaucoma | 108 | 108 | 9.20 ± 1.60 | 536.00 ± 61.00 |
| Control | 24 | 24 | 10.80 ± 1.80 | 550.00 ± 36.00 | |
| Xu, 2011(24) | Glaucoma | 60 | 60 | 9.61 ± 1.56 | 541.40 ± 37.46 |
| Control | 60 | 60 | 10.40 ± 1.62 | 541.75 ± 26.07 | |
| Abitbol, 2010(25) | Glaucoma | 58 | 58 | 8.77 ± 1.40 | 535.34 ± 42.70 |
| Control | 75 | 75 | 10.46 ± 1.60 | 560.20 ± 36.30 | |
| Mangouritsas, 2009(26) | Glaucoma | 108 | 108 | 8.95 ± 1.27 | 526.77 ± 35.73 |
| Control | 74 | 74 | 10.97 ± 1.59 | 537.84 ± 41.93 | |
| Sullivan-Mee, 2008(27) | Glaucoma | 99 | 99 | 8.10 ± 1.50 | 541.00 ± 41.00 |
| Control | 71 | 71 | 9.70 ± 1.50 | 546.00 ± 33.00 | |
| Villas-Bôas, 2009(28) | Glaucoma | 21 | 38 | 8.90 ± 2.10 | 514.80 ± 41.30 |
| Control | 12 | 24 | 10.20 ± 1.60 | 529.00 ± 45.40 | |
| Beyazyıldız, 2014(29) | Glaucoma | 66 | 66 | 9.10 ± 1.90 | 550.40 ± 36.30 |
| Control | 50 | 50 | 9.60 ± 1.70 | 537.30 ± 38.50 |
CCT= central corneal thickness; CH= corneal hysteresis; No=number; SD= standard deviation.
RESULTS
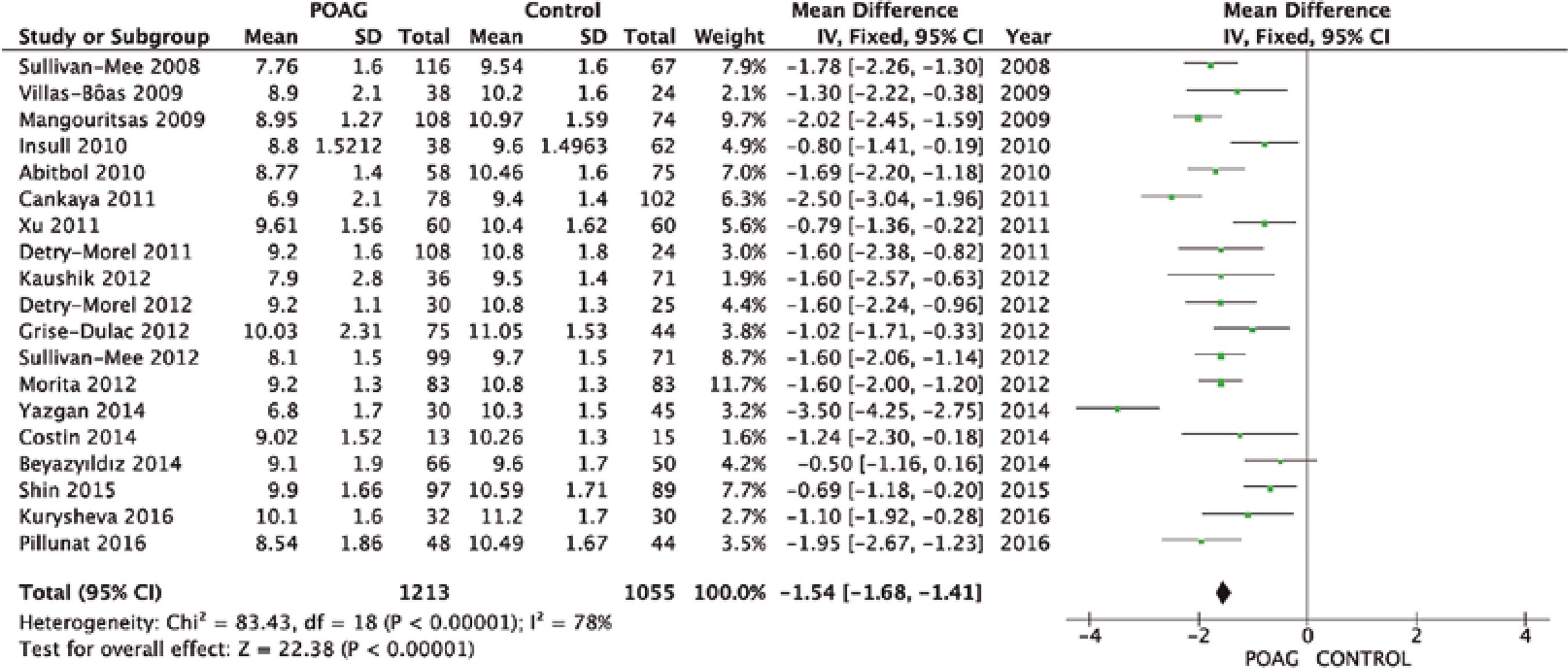
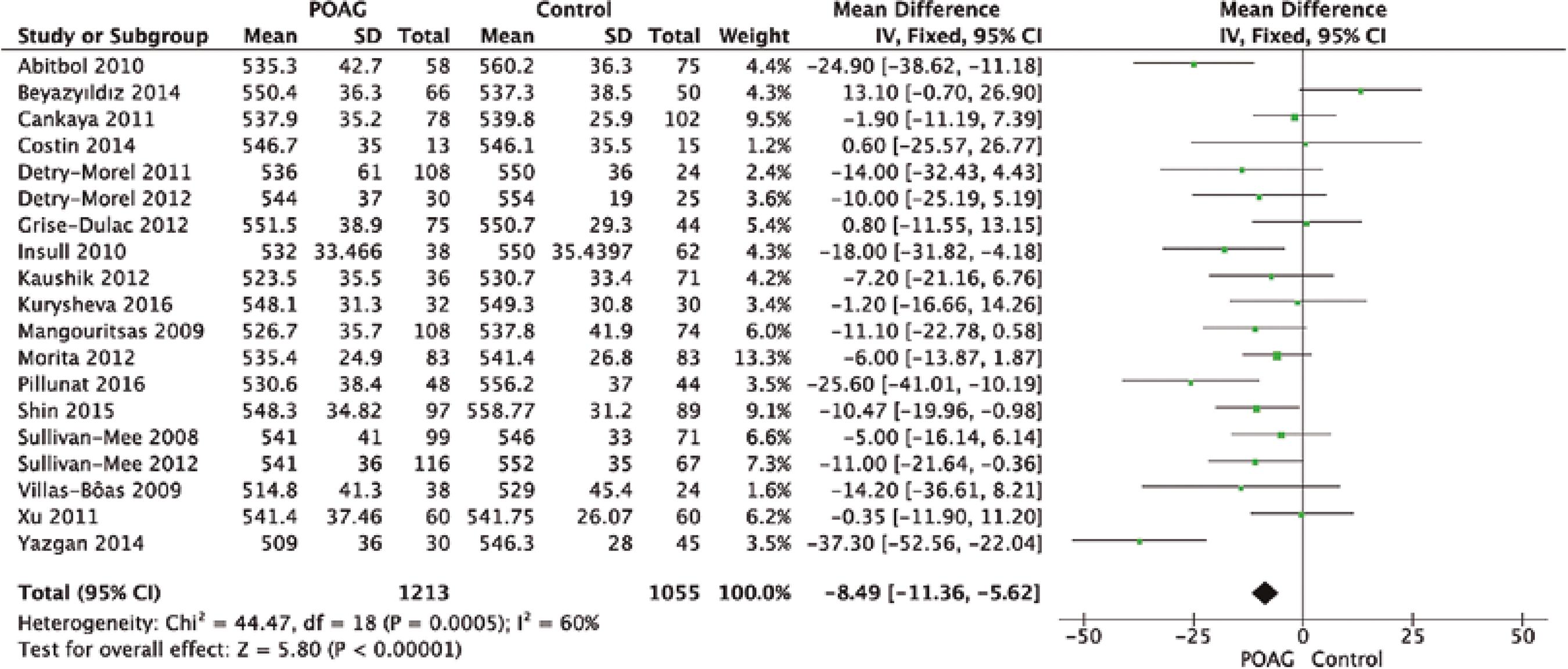
From a total of 124 studies screened, only 19 complied with our eligibility criteria, as shown in figure 1. Table 1 and figures 2 and 3 summarize the mean and standard deviation (SDs) of both CH and CCT for the control and case groups of each study(11-29).
Synthesis of results
A total of 1,213 glaucoma eyes from 1,159 glaucoma patients and 1,055 healthy eyes from 1,021 healthy subjects were considered in our study. Table 1 shows the baseline characteristics of these participants and their eye-related parameters.
Quantitative analysis showed that CH was significantly lower in the glaucoma group than in the control group (MD=-1.54 mmHg, 95% CI [-1.68, -1.41], p<0.00001; Figure 2). Additionally, CCT was significantly lower in the glaucoma group than in the control group (MD=-8.49 µm, 95% CI [-11.36 to -5.62], p<0.001; Figure 3).
DISCUSSION
The latest evidence regarding the true value of CCT as a risk factor for glaucoma is still unclear. While some studies have considered CCT as an important risk factor for the development of glau coma(4-6,10), others such as the EMGT, Barbados Eye Study, and studies by Chauhan et al. and Congdon et al., did not find a simple and linear relationship between CCT and glaucoma(2,4). According to our study, there was a significantly lower CCT value among glaucoma patients than among controls (MD=-8.49 µm, 95% CI [-11.36 to -5.62], p<0.001).
The ORA device provides several biomechanical properties that are assumed to be less influenced by CCT when compared with GAT factors, namely CH, which is a biomechanical property related to the viscoelasticity of the cornea. According to our results, there was a significantly lower CH among glaucoma patients than among controls (MD=-1.54 mmHg, 95% CI [-1.68, -1.41], p<0.00001), and this finding is consistent with previous results from other studies(2,9). As the ORA is a non-contact tonometer(2,3,5,9), parameters measured using this device may be more reliable than those measured using GAT(2,3).
From these study results, a relevant question that arises involves the applicability of CH as an instrument in clinical practice and its reliability. Standard CCT measurements have been widely used and may help interpret IOP findings. However, currently it remains undetermined whether this variable per se is useful for assessing a patient's risk for developing glaucoma. In this sense, by providing further information about corneal biomechanics, CH may differ from CCT. However, there is not enough consolidated evidence to allow replacement of CCT with other markers, such as CH, in the management of glaucoma patients. The fact that CH is not theoretically influenced by CCT(3,5,8,9), which shows large variability in the overall population(5-7), is very important. Thus, it can become a valuable tool in various assessments, such as assessment of the stratification risk of glaucoma patients and even prognosis. However, the ORA is not commonly found in ophthalmology clinics worldwide, and this limits the knowledge of CH in glaucoma.
The results of our study provide strong evidence on the use of CCT and CH. Furthermore, it should be pointed out that this is the first review of the literature and meta-analysis on the topic of CH in glaucoma. However, we recognize that our study has some limitations. We included several glaucoma diagnoses (POAG), normal tension glaucoma [NTG], pre-perimetric POAG, pseudoexfoliative glaucoma [PEXG], and exfoliative glaucoma [EXG]), which could have biased the results, as the different physiopathology of each type of glaucoma may have a different impact on the cornea. Because PubMed was used as the search engine, the included papers were limited to biomedical literature indexed in the MEDLINE database. Additionally, our study only considered articles between 2008 and 2016, according to strict criteria. Furthermore, the ORA device was only introduced in 2005. Therefore, this study had a relatively short review period. Finally, we recognize some other limitations, including the fact that this was not a systematic review, risk of bias evaluation was not performed, and only observational studies were considered to eliminate the risk of bias from interventions.
In conclusion, this study reveals a significant difference in CH and CCT between glaucoma patients and healthy controls. These results indicate that there may be better assessments beyond CCT measurement alone. Therefore, it is important to keep searching for new and more sophisticated tools to measure corneal properties such as CH in order to deepen our knowledge of this subject.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

