INTRODUCTION
Aging is a universal and inexorable biological process that results in a progressive loss of functional reserve in each body organ throughout life(1). Population aging is both a cause and consequence of remarkable social changes that must be properly understood when planning public policies(2). The number of people worldwide older than 60 years is now approximately 600 million, and this number is expected to reach 2 billion by the year 2050, mostly due to people living in industrialized countries(3). Brazil comprises 2.5% of all elderly people in the world(4), and it is expected to have the sixth largest elderly population by the year 2025 (13.8% of its population(5)).
Even though the population older than 80 years is growing fast(2,3), most publications have considered samples up to 80 years old(6-11). A previous study in a small group of centenarians emphasized de mographic characteristics such as female gender, good general health, arterial hypertension, reading difficulties, and visual impairment due to age-related macular degeneration (AMD)(12). To date, however, the relationship between very elderly population, the im pact of low vision, and the psychological impact on health-and vision-related quality of life has not been extensively studied(2,3,13,14). Nevertheless, it is important to understand these relationships for planning and execution of adequate interventions and health policies.
The purpose of the study was to investigate the frequency of eye conditions along with the occurrence and main causes of visual impairment and blindness in the very elderly. We specifically considered how these abnormalities might affect quality of life and vision.
METHODS
This was a cross-sectional observational study performed between April 2007 and July 2008. The research ethics committee of Universidade Federal de São Paulo (UNIFESP) approved the study protocol, which adhered to the tenets of the Declaration of Helsinki and Resolution 196/96 of the Ministry of Health, Brazil. Informed consent was obtained from all participants after explaining the nature of the study and the potential consequences of examination.
Study population
Subjects aged 80 years and older were recruited from three distinct sources: the Department of Ophthalmology, UNIFESP; the Center for Studies in Aging (project EPIDOSO, UNIFESP); and the Albert Einstein Residential Senior Community. All patients were included pro vided they were in good general health to allow examination in the clinic, and provided they could fully understand the written informed consent form. The enrolled participants were grouped into three categories by age: 80-89 years, 90-99 years, and ≥100 years.
Procedures
Individual interviews were performed to obtain demographic, ge neral health, and medical history details. The ophthalmic exam in cluded measurement of the following: presenting visual acuity with glasses when used (the distance at a 6 m projected Snellen optotypes), refraction, measurement of best-corrected visual acuity for distance (measured after refraction testing with the best optical correction), and tear break-up time. We also performed anterior segment biomicroscopy, applanation tonometry with a Goldmann tonometer, and indirect ophthalmoscopy. All exams were executed by the same ophthalmologist (MC). When necessary, supplementary tests were performed to confirm diagnosis, including optical coherence tomography, fluorescein angiography, ultrasound biomicroscopy, and vi sual field testing.
Visual status was stratified as follows, considering visual acuity in the best-seeing eye: normal vision as ≥20/30; mild visual impairment as <20/30 to ≥20/60; moderate visual impairment as <20/60 to ≥20/200; severe visual impairment as <20/200 to ≥20/400; and blindness as <20/400(15-17). Eyes with a presenting visual acuity of ≤20/40 were assigned a principal cause of visual impairment/blindness. Refractive error was assigned as the cause for those eyes where distance visual acuity improved to 20/30 or better with refractive correction. Cataract was diagnosed when lens opacity was commensurate with the altered visual acuity. Age-related macular degeneration was classi fied as determined in the Age-Related Eye Disease Study (AREDS)(18). Glaucoma was diagnosed when at least two of the following events were present: family history of glaucoma, previous diagnosis of glaucoma (with or without anti-hypertensive ocular medication), elevated intraocular pressure (IOP), glaucomatous disc changes, and glaucomatous visual field abnormalities(16).
Two questionnaires were administered by interview: Quality of Life Short Form-36 (SF-36) and the Visual Function Questionnaire (VFQ-25). The SF-36 is a practical and reliable instrument that gives valid information on functional health and wellbeing from the patient's point of view, with an eleven-question instrument and eight dimensions of health/wellbeing that comprise physical functioning, physical role limitation, bodily pain, general health, vitality, social role functioning, emotional functioning, and mental health(19-21). The Portuguese-adapted version of the VFQ-25 (translated and validated in Portuguese) was used in this study, consisting of 12 domains: general health, general vision, near vision, distance vision activities, ocular pain, vision-related social function, vision-related role function, vision-related mental health, vision-related dependency, driving difficulties, color vision, and peripheral vision(22,23). Both questionnaires are standardized on a 0-100 scale, where 0 is the worst and 100 the best. The same interviewer (MC) administered both instruments in the same visit before clinical ophthalmic assessment.
Statistical analysis
Snellen visual acuity measurements were converted to the logarithm of the minimum angle of resolution (logMAR). The correlations between observations in right and left eyes were assessed for the following variables: break-up time, intraocular pressure, and cup-disk ratio(24). When results between eyes were very similar (no statistic variation) the right eye only was selected for analysis(25). Comparisons among groups were done with the Kruskal-Wallis one-way analysis of variance by ranks or the Mann-Whitney U test, as appropriate. Multiple comparison tests were used to identify significant pairwise differences between visual status categories per SF-36 and VFQ-25 domain. P-values <0.05 were considered statistically significant. All analyses were conducted using the SPSS for Windows, Version 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Participants
A total of 150 elderly participants were enrolled and assigned into the three age categories: 80-89 years (70 participants), 90-99 years (50 participants), and ≥100 years (30 participants). For the older group, we had previously reported the results for visual acuity and ocular findings for 20 individuals, but without the data about quality of life (SF-36 questionnaire) or quality of vision (VFQ-25)(12). The demographic features of all participants are shown in table 1. Most were female (n=103; 68.6%) and white (n=121; 80.6%). The most frequent systemic disease was arterial hypertension (n=67; 44.7%), followed by osteoarticular disease (n=21; 14%), while diabetes was reported by 15 (10%; none in the centenarian group). Another 30 participants (20%) claimed to have no disease.
Table 1 Demographic characteristics of the participants
| Group total sample | 80-89 years | 90-99 years | ≥100 years | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=70) | (N=50) | (N=30) | (N=150) | ||||||||
| N | % | N | % | N | % | N | % | ||||
| Gender | |||||||||||
| Female | 43 | 61.43 | 35 | 70.00 | 25 | 83.33 | 103 | 68.67 | |||
| Males | 27 | 38.57 | 15 | 30.00 | 5 | 16.67 | 47 | 31.33 | |||
| Skin color | |||||||||||
| White | 55 | 78.57 | 40 | 80.00 | 26 | 86.67 | 121 | 80.67 | |||
| Black | 7 | 10.00 | 5 | 10.00 | 3 | 10.00 | 15 | 10.00 | |||
| Asian | 3 | 4.29 | 1 | 2.00 | 1 | 3.33 | 5 | 3.33 | |||
| Brown | 5 | 7.14 | 4 | 8.00 | 0 | - | 9 | 6.00 | |||
| Origin | |||||||||||
| Brazil | 55 | 78.57 | 30 | 60.00 | 23 | 76.67 | 108 | 72.00 | |||
| Other country | 15 | 21.43 | 20 | 40.00 | 7 | 23.33 | 42 | 28.00 | |||
| Recruitment | |||||||||||
| Geriatrics outpatient clinic | 56 | 80.00 | 16 | 32.00 | 0 | - | 72 | 48.00 | |||
| Advertisement | 8 | 11.43 | 23 | 46.00 | 29 | 96.67 | 60 | 40.00 | |||
| Residential senior community | 6 | 8.57 | 11 | 22.00 | 1 | 3.33 | 18 | 12.00 | |||
Visual acuity
Graphic 1 shows the presenting and best-corrected visual acuities. Presenting normal vision was found in 20 participants (13.3%), with mild visual impairment in 53 (35.4%), moderate visual impairment in 50 (33.3%), severe visual impairment in 8 (5.3%), and blindness in 19 (12.7%). With best-correction visual acuity, normal vision was present in 37 participants (24.7%), mild visual impairment in 55 (36.7%), moderate visual impairment in 38 (25.3%), severe visual impairment in 5 (3.3%), and blindness in 15 (10%). None of the participants had 20/20 PVA in the best-vision eye, irrespective of the age group.
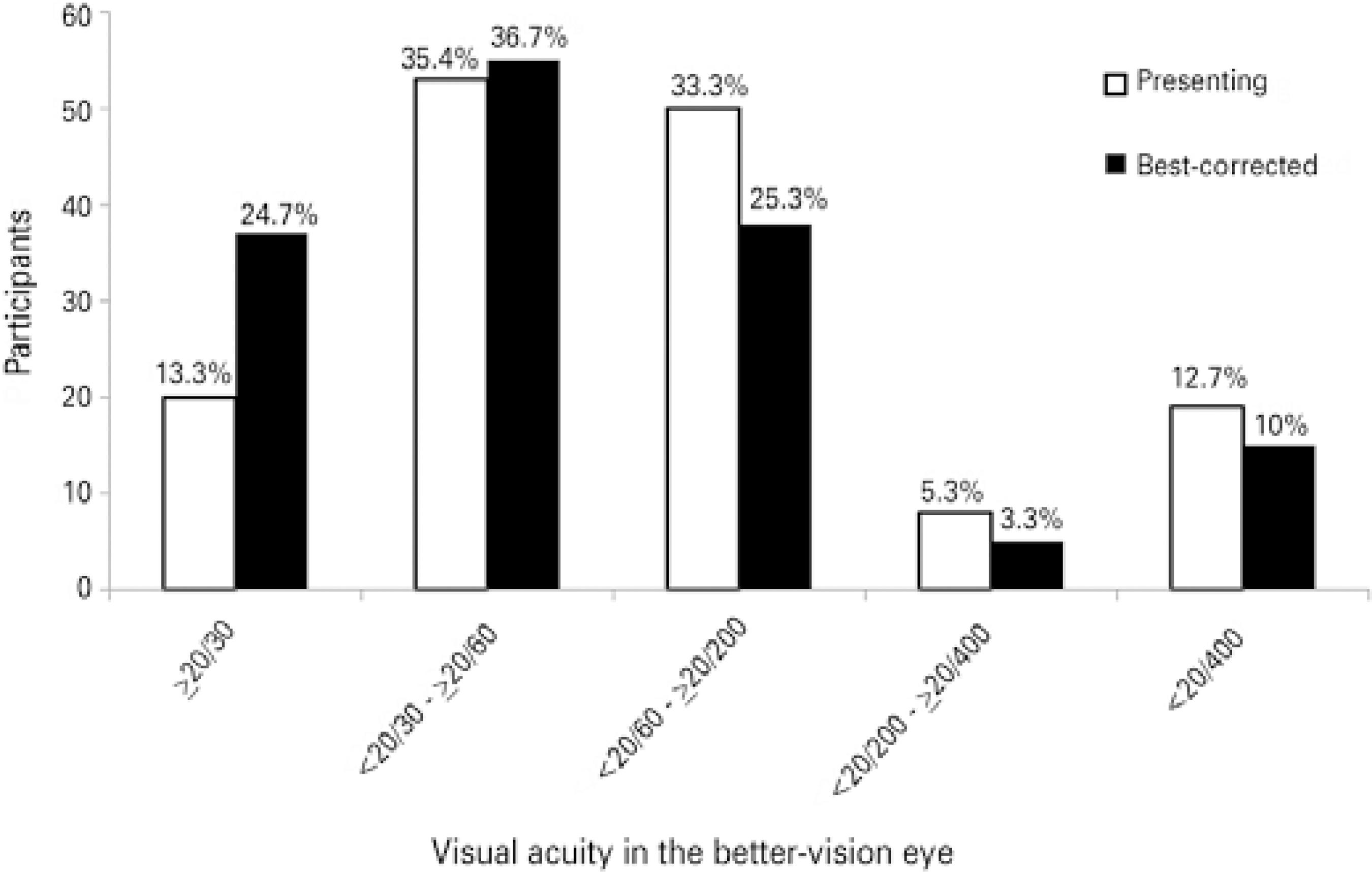
Graphic 1 The distribution of presenting and best-corrected visual acuity by the following distance visual acuity categories in best-seeing eyes: normal vision (≥20/30), mild visual impairment (<20/30 to≥ 20/60), moderate visual impairment (<20/60 to ≥20/200), severe visual impairment (<20/200 to ≥20/400), and blindness (<20/400)(15,17).
Ocular findings
Ocular findings per eye for the three age groups are shown in table 2. On the anterior segment, the most frequent finding was arcus senilis (n=226; 75.3%), followed by eyelid disorders (n=218; 72.6%). Lens status per eye was classified as present with some degree of opacity in 199 eyes (66.3%), pseudophakic in 82 eyes (27.3%), aphakic in 6 eyes (2.0%), and replaced by an ocular prosthesis in 1 eye. In 10 eyes (3.3%), the lens was fully clear. Age-related macular degeneration was present in 240 eyes (80%), hypertensive retinopathy in 40 eyes (13.3%), and diabetic retinopathy in 2 eyes (0.6%).
Table 2 Ocular findings by age groupa
| Age group | 80-89 years | 90-99 years | ≥100 years | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total sample | 140 eyes | 100 eyes | 60 eyes | 300 eyes | |||||||
| N | % | N | % | N | % | N | % | ||||
| Anterior segment findings | |||||||||||
| Arcus senilis | 88 | 62.86 | 80 | 80.00 | 58 | 96.67 | 226 | 75.33 | |||
| Few lashes | 86 | 61.43 | 96 | 96.00 | 36 | 60.00 | 218 | 72.67 | |||
| Pterygium | 4 | 2.86 | 6 | 6.00 | 6 | 10.00 | 16 | 5.33 | |||
| Corneal transplant | 4 | 2.86 | 2 | 2.00 | 0 | 0.00 | 6 | 2.00 | |||
| Disorganized/absent globe* | 1 | 0.71 | 1 | 1.00 | 0 | 0.00 | 2 | 0.67 | |||
| Vitreous touching cornea** | 0 | 0.00 | 1 | 1.00 | 0 | 0.00 | 1 | 0.33 | |||
| Lens status | |||||||||||
| Cataract | 105 | 75.00 | 58 | 58.00 | 36 | 60.00 | 199 | 66.33 | |||
| Pseudophakic | 26 | 18.57 | 38 | 38.00 | 18 | 30.00 | 82 | 27.33 | |||
| Normal | 7 | 5.00 | 2 | 2.00 | 1 | 1.66 | 10 | 3.33 | |||
| Aphakic | 1 | 0.71 | 0 | 0.00 | 5 | 8.33 | 6 | 2.00 | |||
| Undetermined* | 1 | 0.71 | 1 | 1.00 | 0 | 0.00 | 2 | 0.67 | |||
| Posterior segment findings | |||||||||||
| Age-related macular degeneration (AMD) | 90 | 64.29 | 90 | 90.00 | 60 | 100.00 | 240 | 80.00 | |||
| AMD dry early/moderate form | 80 | 57.14 | 74 | 74.00 | 42 | 70.00 | 196 | 65.33 | |||
| AMD dry advanced form | 6 | 4.29 | 16 | 16.00 | 16 | 26.67 | 38 | 12.67 | |||
| AMD wet form | 4 | 2.86 | 0 | 0.00 | 2 | 3.33 | 6 | 2.00 | |||
| Hypertension retinopathy | 14 | 10.00 | 12 | 12.00 | 14 | 23.33 | 40 | 13.33 | |||
| Myopic degeneration | 14 | 10.00 | 8 | 8.00 | 4 | 6.67 | 26 | 8.67 | |||
| Diabetic retinopathy | 0 | 0.00 | 2 | 2.00 | 0 | 0.00 | 2 | 0.67 | |||
aMore than one ocular finding identified per person.
*An 89-year-old female with phthisis bulbi in the right eye, and a 90-year-old female with ocular prosthesis in the right eye.
**A 98-year-old female with vitreous in the anterior chamber (complication from previous cataract surgery without intraocular lens implantation).
Refractive errors were found in 68 participants (45.3%), which included 41 (58.6%) aged 80-89 years, 13 (26%) aged 90-99 years, and 14 (46.6%) aged ≥100 years. Glasses for distance were used by 91 participants (60.7%), and 105 (70.0%) required additional lenses.
Break-up time less than 10 seconds was noted in 95 (63.3%) patients. Intraocular pressure (IOP) ranged from 6 to 28 mmHg in the group aged 80-89 years, with a mean of 13.7 mmHg (SD 3.64) and 3 individuals in this group having an IOP ≥20 mmHg. In the group aged 90-99 years, IOP ranged from 9 to 18 mmHg, with a mean of 12.4 ± 3.84 mmHg. In the centenarian group, IOP ranged from 7 to 28 mmHg, with a mean of 12.8 ± 4.77 mmHg, with 1 having an IOP ≥20 mmHg. Glaucoma was diagnosed in 18 participants (12.0%), and 6 (4.0%) had previously had glaucoma surgery.
Principal causes of visual impairment/blindness
The principal causes of visual impairment/blindness are summarized in table 3 and graphic 2 (for the visual status of 130 individuals with mild visual impairment or worse). Overall, cataract was the most frequent cause of visual impairment (n=57; 43.8%), followed by uncorrected refractive error (n=28; 21.5%), AMD (n=23; 17.7%), and myopic degeneration (n=5; 3.8%); glaucoma was described as the principal cause of visual impairment/blindness in 3 participants (2.3%).
Table 3 Principal causes of visual impairment/blindness by age group (years)
| Mild (<20/30 to ≥20/60) | Moderate (≤20/60 to ≥20/200) | Severe (<20/200 to ≥20/400) | Blindness (<20/400) | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 80-89 | 90-99 | ≥100 | 80-89 | 90-99 | ≥100 | 80-89 | 90-99 | ≥100 | 80-89 | 90-99 | ≥100 | |||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||||
| Cataract | 13 ( 40.6) | 7 ( 36.8) | 1 ( 50.0) | 8 ( 47.1) | 9 ( 42.9) | 6 ( 50.0) | 1 ( 50.0) | 2 ( 50.0) | 2 (100.0) | 3 ( 75.0) | 2 ( 33.3) | 3 ( 33.3) | 57 ( 43.8) | |||
| Refractive error | 15 ( 46.9) | 3 ( 15.8) | - | 5 ( 29.4) | 4 ( 19.0) | - | 1 ( 50.0) | - | - | - | - | - | 28 ( 21.5) | |||
| AMD | 3 ( 9.4) | 6 ( 31.6) | - | 1 ( 5.9) | 3 ( 14.3) | 4 ( 33.3) | - | 1 ( 25.0) | - | - | 2 ( 33.3) | 3 ( 33.3) | 23 ( 17.7) | |||
| Myopic degeneration | 1 ( 3.1) | - | - | 1 ( 5.9) | 2 ( 9.5) | - | - | 1 ( 25.0) | - | - | - | - | 5 ( 3.8) | |||
| Maculopathy | - | 1 ( 5.3) | - | 1 ( 5.9) | - | 1 ( 8.3) | - | - | - | - | 1 ( 12.7) | - | 4 ( 3.1) | |||
| Aphakia | - | - | - | - | - | 1 ( 8.3) | - | - | - | - | 1 ( 12.7) | 1 ( 11.1) | 3 ( 2.3) | |||
| Glaucoma | - | - | - | - | 2 ( 9.5) | - | - | - | - | - | - | 1 ( 11.1) | 3 ( 2.3) | |||
| ODP | - | - | 1 ( 50.0) | 1 ( 5.9) | - | - | - | - | - | - | - | - | 2 ( 1.5) | |||
| Keratitis | - | 1 ( 5.3) | - | - | - | - | - | - | - | - | - | - | 1 ( 0.8) | |||
| PCO | - | 1 ( 5.3) | - | - | - | - | - | - | - | - | - | - | 1 ( 0.8) | |||
| DR | - | - | - | - | 1 ( 4.8) | - | - | - | - | - | - | - | 1 ( 0.8) | |||
| CAO | - | - | - | - | - | - | - | - | - | - | - | 1 ( 11.1) | 1 ( 0.8) | |||
| CVO | - | - | - | - | - | - | - | - | - | 1 ( 25.0) | - | - | 1 ( 0.8) | |||
| Total | 32 (100.0) | 19 (100.0) | 2 (100.0) | 17 (100.0) | 21 (100.0) | 12 (100.0) | 2 (100.0) | 4 (100.0) | 2 (100.0) | 4 (100.0) | 6 (100.0) | 9 (100.0) | 130 (100.0) | |||
The results are for the presenting visual acuity in the eye with the best vision. AMD= age-related macular degeneration; ODP= optic disk pallor; PCO= posterior capsule opacity; DR= diabetic retinopathy; CAO= central arterial occlusion; CVO= central vein occlusion.
Quality of life and vision-related quality of life
Of the 150 participants, 137 were competed interviews for both the SF-36 and VFQ-25; the other 13 non-respondents included 2 from group aged 80-89 years, 1 from the group aged 90-99, and 10 from the centenarian group. Major reasons for non-response/partial interviews were obstacles to comprehension, including severe hearing loss and/or unreliable information.
SF-36 scores by visual status, and per domain, are shown in table 4. In general, participants with normal presenting visual acuity had significantly higher scores for the following domains: physical functioning, physical/role limitation, general health, vitality, social role functioning, emotional role functioning, and mental health. No statistical differences were found among the five visual status categories for the bodily pain domain.
Table 4 SF-36 scores by visual status
| Domain | Normal | Mild visual impairment | Moderate visual impairment | Severe visual impairment | Blindness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Physical functioning | 73.49* | 21.20 | 55.80* | 28.51 | 50.89* | 25.93 | 33.33* | 24.22 | 34.69* | 30.08 | ||||
| Physical role limitation | 72.09* | 45.39 | 56.25* | 49.75 | 70.54* | 45.67 | 16.67* | 40.82 | 31.25* | 47.87 | ||||
| Bodily pain | 79.72 | 23.53 | 70.73* | 24.68 | 78.75* | 20.61 | 73.33* | 13.60 | 70.06* | 27.47 | ||||
| General health | 76.05* | 19.01 | 67.86* | 14.93 | 68.25* | 17.56 | 68.83* | 18.13 | 58.56* | 22.41 | ||||
| Vitality | 72.33* | 17.94 | 58.41* | 19.04 | 56.96* | 16.57 | 74.17# | 13.93 | 46.25* # | 25.00 | ||||
| Social role functioning | 74.07* | 26.11 | 60.51* | 27.06 | 60.09* | 24.59 | 69.17* | 20.84 | 46.41* | 30.18 | ||||
| Emotional role functioning | 65.89* | 47.41 | 51.52* | 50.04 | 59.52* | 49.16 | 33.33* | 51.64 | 18.75* | 40.31 | ||||
| Mental health | 68.09* | 20.72 | 63.00* | 18.82 | 62.14* | 19.40 | 73.33* | 15.73 | 50.75* | 19.80 | ||||
*statistical significance determined by post-hoc multiple comparison test (P<0.05) for the normal visual acuity category when compared to each other is marked with the symbol *for the same domain.
#statistical significance determined by post-hoc multiple comparison test (P<0.05) for the severe visual impairment category when compared to each other is marked with the symbol # for the same domain.
SD= standard deviation; SF-36= Quality of Life Short Form-36.
Table 5 shows the VFQ-25 scores by visual status category, per domain. Subjects with normal vision presented significantly higher scores for the domains general health and general vision. No statistical differences were found among the five visual status categories for the ocular pain domain. Distance activities, social functioning, mental health, role difficulties and dependency domains presented significantly higher scores in those with normal visual acuity and all other categories except mild visual impairment (normal versus either moderate visual impairment, severe visual impairment, or blindness; all p=0.001). No statistical differences were found among the five visual status categories for the driving domain.
Table 5 VFQ-25 scores by visual status
| Domain | Normal | Mild visual impairment | Moderate visual impairment | Severe visual impairment | Blindness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| General health | 56.98* | 24.60 | 40.34* 0 | 20.33 | 25.00* 0 | 22.57 | 33.33 | 20.41 | 12.50* 0 | 15.81 | ||||
| General vision | 83.72* | 15.89 | 68.18* 0 | 21.27 | 59.29* # | 19.99 | 53.33* | 20.66 | 36.25* 0 # | 16.68 | ||||
| Ocular pain | 46.51* | 7.38 | 46.59 | 10.57 | 53.13 | 13.45 | 56.25 | 10.46 | 53.91 | 14.23 | ||||
| Distance activities | 96.90* | 7.50 | 86.930 | 17.37 | 72.47* 0 # | 25.59 | 37.50* 0 # | 33.23 | 36.72* 0 # | 27.27 | ||||
| Social functioning | 99.71* | 1.91 | 95.740 | 11.73 | 85.71* # | 22.75 | 52.08* 0 # | 37.43 | 56.25* 0 # | 31.95 | ||||
| Mental health | 92.44* | 10.07 | 85.090 | 15.85 | 70.09* 0 # | 22.97 | 58.33* 0 | 26.12 | 38.67* 0 # | 25.13 | ||||
| Role difficulties | 96.80* | 8.23 | 85.510 | 20.87 | 67.41* 0 # | 28.33 | 41.67* 0 # | 27.00 | 30.47* 0 # | 19.35 | ||||
| Dependency | 98.64* | 4.79 | 88.830 | 18.23 | 77.08* | 28.20 | 52.78* 0 | 35.22 | 38.54* 0 | 25.07 | ||||
| Driving | 90.00* | 13.69 | 50.00 | 39.53 | 25.00 | 50.00 | NR | - | NR | - | ||||
| Color vision | 100.00* | 0.00 | 97.730 | 11.83 | 89.29# | 19.75 | 50.00* 0 # | 41.83 | 54.69* 0 # | 37.88 | ||||
| Peripheral vision | 99.42* | 3.81 | 93.180 | 14.63 | 82.14* # | 24.40 | 41.67* 0 # | 40.82 | 45.31* 0 # | 40.02 | ||||
| Near activities | 93.75* | 11.79 | 60.03* | 37.81 | 45.71* | 36.92 | NR | - | NR | - | ||||
*(P<0.05) among the normal visual acuity category when compared to each other is marked with the symbol; *= for the same domain (determined by post-hoc multiple comparison test).
0(P<0.05) among the normal visual acuity category when compared to each other is marked with the symbol; 0= for the same domain (determined by post-hoc multiple comparison test).
#(P<0.05) among the severe visual impairment category when compared to each other is marked with the symbol; #= for the same domain (determined by post-hoc multiple comparison test). SD= standard deviation; NR= no response; VFQ-25= visual function questionnaire.
The color vision domain showed significantly higher scores when comparing normal vision to severe visual impairment (p=0.001) and to blindness (p=0.001). Significantly higher scores were found in the normal vision group when compared with the moderate visual impairment (p=0.009), severe visual impairment (p=0.001), and blindness (p=0.001) groups for the peripheral vision domain. In the near activities domain, significantly higher scores were found for the normal vision group when compared with the mild (p=0.001) and moderate (p=0.001) visual impairment categories.
DISCUSSION
This study comprised a large sample of very elderly participants, even though it was only a convenience sample. In general, the participants were in good health, which contrasts to that usually seen with patients living in nursing homes or who have cognitive dysfunction.
The care of the very old may be neglected by family members and, as a result, damaged or antiquated eyeglasses may not be replaced in a timely fashion. Besides a lack of attention from the family, very old individuals often assume that vision loss is a natural consequence of aging(26). Clinically, regardless of the expected physiological changes in vision, full attention should be given to those with visual impairment/blindness(1). Our study showed, however, that a number of participants did achieve better vision after adequate refractive correction. Correcting refractive errors improves potential vision, and can stimulate wider contact, independence, and a social life, thereby helping age-related quality of life.
As confirmed in this study, it is known that visual acuity decreases as age increases(6,8,9,27). The age-related decline in visual performance may be explained in terms of reductions in the illuminance of the visual stimulus due to changes in the ocular media and losses of efficiency at a neural level(26). Such physiological modifications may explain why none of the patients in this sample had a presenting visual acuity of 20/20.
Most causes of reversible low vision can be treated easily. This is particularly true for uncorrected refractive errors(28). In this study, 20% of participants achieved better visual acuity for distance with appropriate correction. After refractive correction, the frequency of visual impairment decreased from 33.3% to 25.3%, while that for blindness decreased from 12.7% to 10%. In all three age groups, individuals achieved better vision with proper spectacles, even when they had AMD and early cataracts, except for those with glaucoma.
AMD affected over half of the patients aged 80-89 years, most of those aged 90-99 years, and all of those aged ≥100 years, a trend that is consistent with previous studies(7,8,29). Although AMD was present in all centenarians, when stratified by severity and different forms, most had an initial atrophic form of the disease that was sometimes not even considered the primary cause of low vision. A study of very old adults in Iceland reported that AMD was present in 54% of those aged ≥75 years, 64% of those aged ≥85 years, 74% of those aged ≥95 years, and 100% of those aged ≥100 years(29).
Glaucoma was detected in 12% of the sample: 14.3% aged 80-89 years, 12% aged 90-99 years, and 8% aged ≥100 years. In general, glaucoma prevalence varies by the population studied, ranging from 3% to 14% (average 7%-9%) in most studies, but rising with age(9-11,16,28). The large variance in prevalence may result from the use of different diagnostic criteria among the studies. Surprisingly, though, our results revealed that rates decrease as age increases, and we are uncertain as to why this occurred. We posit that it might be due to selection bias.
The main causes of low visual acuity in the study were cataract followed by uncorrected refractive error, AMD, myopic degenera tion, and glaucoma. These findings corroborate previous studies(6,7,9,10,16,27,28,30). Cataract is described as an important cause of low visual acuity. Its prevalence as a cause of visual impairment/blindness increased consistently with age and severity of vision impairment(28,30). Uncorrected refractive errors were also an important cause of low vision, especially in the younger age groups and in those with mild to moderate visual impairment. In other studies, AMD was the third main cause of visual impairment, usually being the primary cause of low vision in studies from industrialized countries(15,17). This finding provides a basis for the requirement that public policies are needed to ensure early diagnosis and adequate treatment for the very old in Brazil.
Health promotion is an important goal in the elderly, and can positively affect health-related quality of life(21). Visual impairment detrimentally interferes with daily functions and social activities, significantly affecting health-related quality of life(19,21). The SF-36 is an efficient and validated measure for use in the elderly community, and using the interviewer- administered version improves completion rates(19). The perception of quality of life, as measured by the SF-36, was worse in elderly patients with low visual acuity, similar to the results described in other studies of elderly populations(19,20). This finding can be explained by the fact that visual impairment reduces physical activity, independent mobility, and social functioning, each of which are investigated in the SF-36 domains. The VFQ-25 scores for each domain were consistently lower in participants with lower visual acuity results, similar to those described in another study(22). The near VFQ-25 domain presented higher scores in the elderly who had the best visual acuity, irrespective of age, emphasizing the importance of near vision for elderly participants. When adversely affected, near vision interferes directly with perception of vision quality. The information obtained by questionnaires on quality of life can help determine the type of care needed to support the independence and improve the quality of daily life among the elderly population(20,22).
It is important to note that the association between visual impairment and decreased function and wellbeing are integral to a person's health-related quality of life; moreover, it is not easy to isolate these factors from other medical conditions, as observed in this and other studies(20). In agreement with other publications, the use of questionnaires that consider self-reported functioning and wellbeing can provide data of the effect of disease on daily activities from the patient perspective(20,22). When considering visual status, it can give information about the direct influence of reduced visual acuity on health-related quality of life(1,22). Visual impairment is a particular risk factor for isolation, depression, and falls(1,22). These facts affect the elderly at both individual and societal levels, resulting in greater use of healthcare services despite remaining active and productive. Depending on others for activities of daily life may necessitate increased expenditure for the elderly themselves, their family, and wider society in general. Special care and attention should be given to the vision of the very old, aiming to prevent or diagnose ocular disorders early.
In the very old, we showed that AMD was very frequent, affecting 100% of all centenarians. Cataracts were also highly prevalent, with more than a third of the sample having previously undergone cataract surgery. Adequate spectacle correction is always desirable in this population, even when a small improvement in visual acuity is achieved, because small changes in image quality might significantly affect daily activities. The negative impact of visual impairment and blindness was confirmed by decreased scores in health- and vision-re lated quality of life instruments. We conclude that the shift in developing countries' populations toward older ages justifies high quality eye care to ensure best vision and quality of life.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

