INTRODUCTION
Ocular allergy is characterized by an inflammatory reaction of the ocular surface caused by hypersensitivity reactions type I or IV. Disease severity is related to the magnitude of the resulting inflammation and is influenced by the patient's age, as well as genetic and environmental factors. Types of ocular allergy include seasonal conjunctivitis, perennial conjunctivitis, atopic keratoconjunctivitis, and vernal keratoconjunctivitis (VKC)(1).
VKC is a chronic, bilateral (though at times asymmetrical), seaso nally exacerbated, allergic inflammation of the tarsal conjunctiva, bulbar conjunctiva, or both. It is more common in children and young adults with known atopy, and typically presents with pruritus, hyperemia, photophobia, and watering(2). The conjunctival changes in VKC are the most pronounced of the subtypes of ocular allergy, being characterized by the formation of giant papillae in the upper tarsal conjunctiva and by swollen limbal lesions(3). The pathology also differs from seasonal and perennial allergic conjunctivitis, because it is mediated by Th2 lymphocytes (type IV hypersensitivity reaction). However, the precise roles of mast cells, eosinophils, fibroblasts, and their cytokines in the inflammatory and remodeling processes of conjunctival tissue are yet to be established(3). Although conjunctival inflammation typically subsides and corneal lesions typically heal spontaneously beyond adolescence, individuals may be left with corneal scarring, opacities, and irregular astigmatism that can permanently reduce the quality of vision(3).
Broadly, the treatment of VKC is divided into preventive, clinical, and surgical options. Preventive options involve eliminating or avoiding allergens like house dust mites and pollen, while surgical options involve scraping fibrin from non-healing shield ulcers or removing upper tarsal giant papillae; however, surgical options are reserved for severe cases(4). Regarding medical treatment, topical an tihistamines, mast cell stabilizers, and drugs with multiple actions (e.g., alcaftadine, olopatadine) are typical first-line options(4). Sodium cromoglycate is the most commonly used mast cell stabilizer, which acts by blocking the degranulation of inflammatory mediators, thereby inhibiting hypersensitivity reaction type I(5,6). The effects of mast cell stabilizers are noticeable 2 to 5 days after starting treatment, but the maximum effect is only achieved by 15 days. Therefore, sodium cromoglycate is best used to prevent recurrences of VKC after initial disease control. Topical corticosteroids are also useful when disease is severe. However, because steroids are associated with treatment-related adverse effects, such as cataract, glaucoma, and keratitis(7), they should be reserved for the management of acute allergic crises and for no more than 2 to 4 weeks(8,9).
Immunomodulators, such as tacrolimus and cyclosporine, have recently been used as treatment alternatives because of their potent anti-inflammatory effects and favorable side-effect profiles. Indeed, these agents have not only been used to replace corticosteroids for ocular allergic crises but also to replace other agents for the maintenance of controlled VKC. Tacrolimus is an immunosuppressant in the macrolide family, which includes cyclosporine. Its mechanism of action consists of decreasing the production of inflammatory mediators by T lymphocytes through the inhibition of calcineurin, an intracytoplasmic protein essential for interleukin (IL)-2 and IL-4 transcription(10). It has been extensively studied because of its widespread use as an immunosuppressant to control rejection of solid organ trans plants(11) and because it is an effective agent in the treatment of autoimmune skin disorders like atopic dermatitis and vitiligo(12). Furthermore, it has been reported that tacrolimus inhibits histamine release from mast cells, impairs prostaglandin synthesis(13), and suppresses histamine release from basophils(14). These three actions may effectively reduce allergic symptoms in VKC.
An in vitro study has shown that the immunosuppressive effect of tacrolimus is 100-times higher than that of cyclosporine(15). Furthermore, it has been shown to be better tolerated(16). Numerous studies have also described the use of tacrolimus for the treatment of ocular disease, such as corneal graft rejection, Mooren's ulcer, uveitis, and graft-versus-host disease(10,17). Tacrolimus may have similar efficacy to corticosteroids in both the control of allergic crises and in the mainte nance of stable disease, but may benefit from a lower incidence of adverse effects(18,19).
In routine practice, patients with ocular allergy often use a com bination of eye drops, which both increases the cost and compromises treatment compliance. Given this and the putative benefits of tacrolimus, we wanted to evaluate the efficacy of topical monotherapy with tacrolimus compared with sodium cromoglycate for the treatment of VKC.
METHODS
Study design
This study was a prospective randomized double-masked clinical trial conducted at a single tertiary care center in São Paulo, Brazil. We compared the efficacy of topical tacrolimus 0.03% eye drops with sodium cromoglycate 4% eye drops for the treatment of VKC. The study was conducted in accordance with the Declaration of Helsinki and initiated after approval by the Research Ethics Committee of the São Paulo Hospital, Federal University of São Paulo under the CAAE number: 47393715.4.0000.5505.
Patient selection
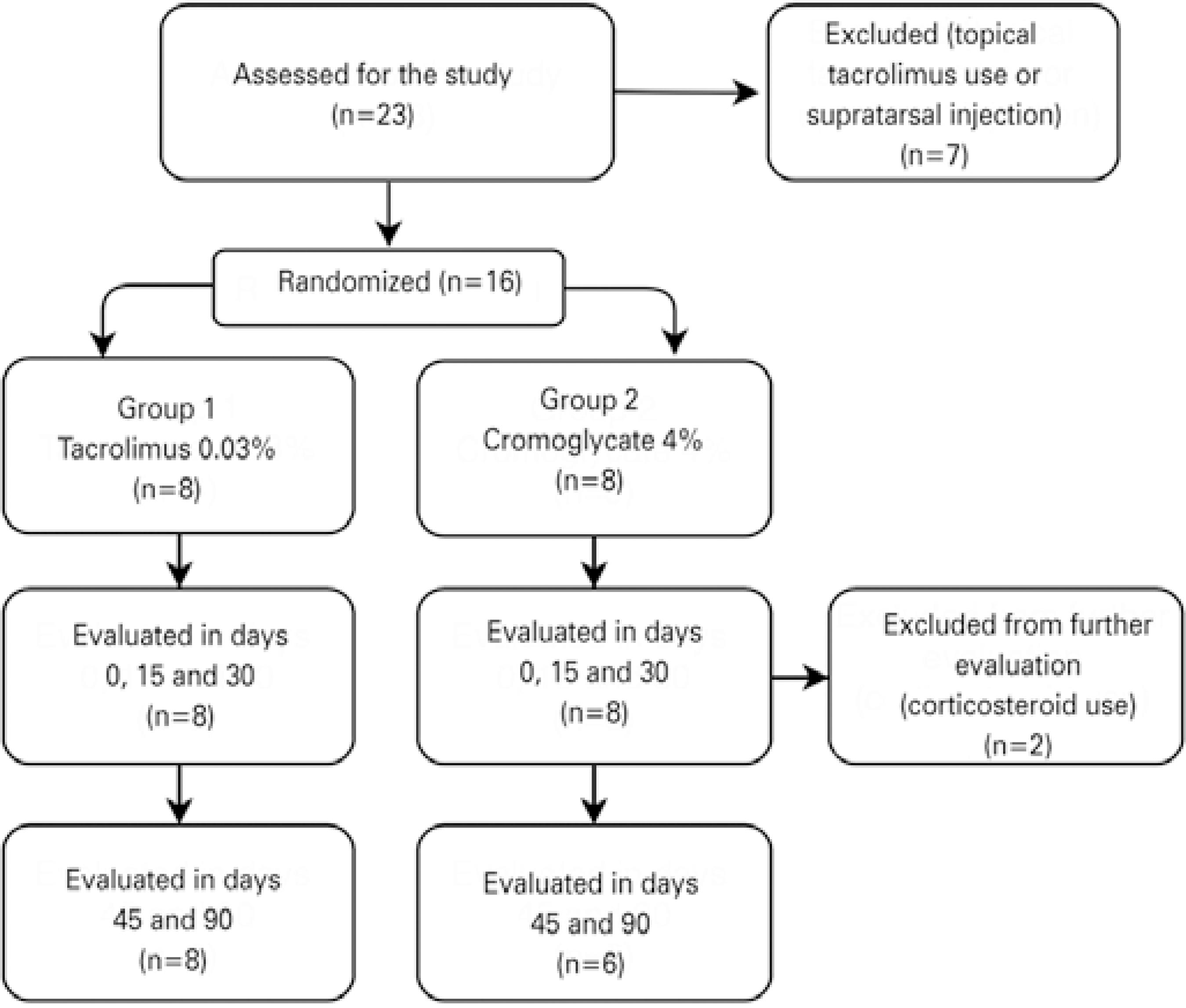
Patients were selected from the External Diseases and Cornea Outpatient Unit and from the Ophthalmic Emergency Care Unit. VKC was diagnosed based on chronic complaints of itching, foreign body sensation, photophobia, and tearing, together with typical findings of limbal inflammatory activity (i.e., limbal hyperemia and Horner-Trantas dots) and giant papillae on the upper tarsal conjunctiva. We excluded patients who were younger than 7 years and older than 18 years, who had current ocular infections, who were using topical tacrolimus, who had ever used a systemic immunosuppressant, or who had undergone supratarsal corticosteroid injection. Patients meeting the criteria for inclusion, together with their legal representatives, were asked to read and agree with informed consent forms.
Trial protocol
Individuals were allocated to two different groups in a 1:1 ratio based on previous block randomization (4 patients per block) with a random number table created using Stata 12® (StataCorp LP, TX, USA). Group 1 was to receive tacrolimus 0.03% eye drops every 8 h and Group 2 was to receive sodium cromoglycate 4% eye drops every 8 h (Both eye drops were obtained from Ophthalmos, São Paulo, Brazil). Patients, healthcare providers, and data collectors were masked to the treatment drug. For double-masking, the eye drop flasks were numbered and contained no identifying marks, and the contents of the flasks were only revealed at the end of the data collection period.
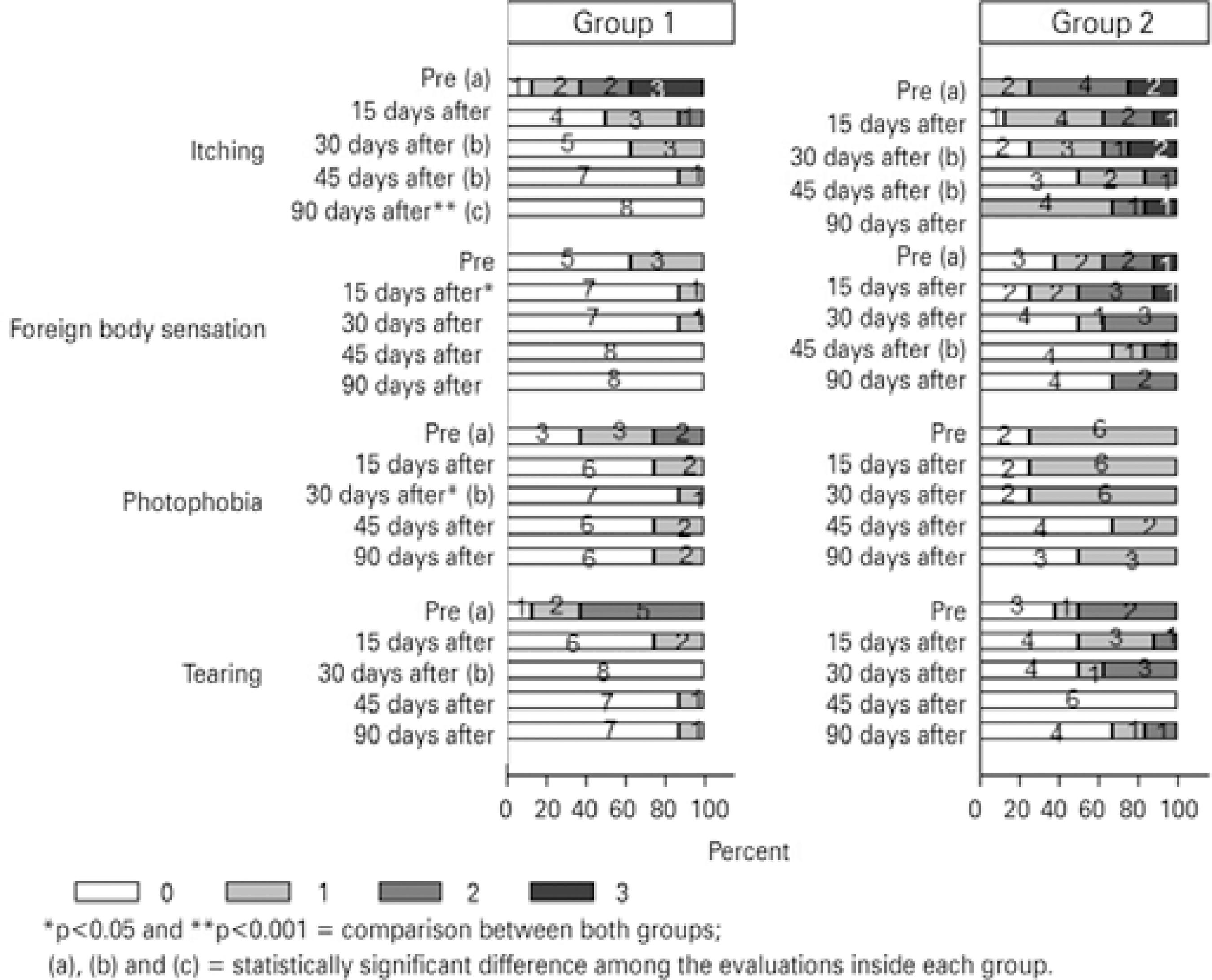
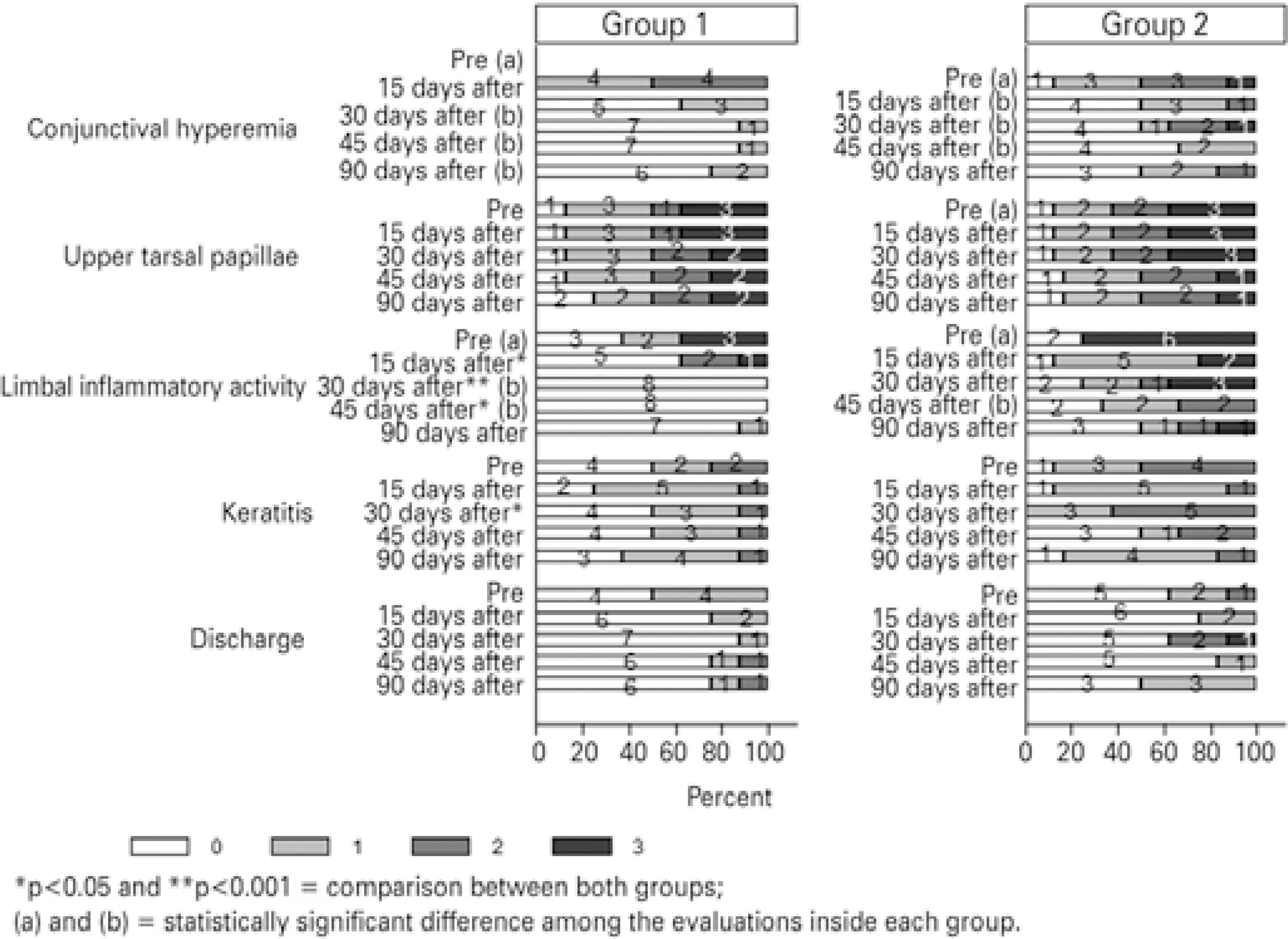
After the initial approach and group assignment, we applied a protocol for the objective assessment of signs and symptoms, adapted from clinical trials with similar methods and objectives(20-22). Symptoms of itching, foreign body sensation, photophobia, and tearing, in addition to signs of conjunctival hyperemia, upper tarsal pa pillae, limbal inflammatory activity, keratitis, and discharge, were assessed and graded by severity from 0 to 3, with higher scores indicating greater severity (Tables 1 and 2). Disease subtype was defined as follows: patients with grade 3 papillae and grade 0 or 1 limbi were classed as having tarsal disease; patients with grade 0, 1, or 2 papillae and grade 2 or 3 limbi were classed as having limbal disease; and all other patients were classed as having mixed tarsal-limbal disease. Symptoms and signs were assessed before and during therapy (i.e., at 0, 15, 30, 45, and 90 days). Variables were analyzed individually, and because of the bilateral nature of VKC, evaluation was based on the examination of both eyes, but data was recorded for the worse eye.
Table 1 Severity scores for symptoms of vernal keratoconjunctivitis
| Symptom | Severity score |
|---|---|
| Itching | 0: absent, no desire to scratch |
| 1+: intermittent desire to scratch | |
| 2+: frequent desire to scratch | |
| 3+: constant desire to scratch | |
| Foreign body sensation | 0: absent, no foreign body sensation |
| 1+: discrete, similar to dust | |
| 2+: mild, similar to sand | |
| 3+: severe, constant, and similar to rock | |
| Photophobia | 0: absent, no photophobia |
| 1+: mild, squints in bright light | |
| 2+: moderate, improves with use of sunglasses | |
| 3+: severe, improves only with total eye occlusion | |
| Tearing | 0: absent |
| 1+: humid, no epiphora | |
| 2+: intermittent epiphora | |
| 3+: constant epiphora |
Table 2 Severity scores for signs of vernal keratoconjunctivitis
| Sign | Severity score |
|---|---|
| Conjunctival hyperemia | 0: absent, calm conjunctiva |
| 1+: mild, increase in vessel diameter, difficult to notice | |
| 2+: moderate, increase in diameter and number of vessels | |
| 3+: diffuse and intense hyperemia | |
| Upper tarsal papillae | 0: absent on the central tarsal conjunctiva |
| 1+: present on the central tarsal conjunctiva | |
| 2+: some giant papillae | |
| 3+: giant papillae predominance | |
| Limbus | 0: no limbal inflammatory activity |
| 1+: limbal hyperemia | |
| 2+: limbal hyperemia and papillae | |
| 3+: Horner-Trantas dots | |
| Keratitis | 0: absent, no epitheliopathy |
| 1+: superficial punctate keratitis | |
| 2+: confluent punctate keratitis | |
| 3+: shield ulcer | |
| Discharge | 0: absent, no discharge |
| 1+: little amount in the fornix | |
| 2+: moderate amount in the fornix | |
| 3+: great amount in the fornix, sticky eyes in the morning |
No group was allowed concomitant treatment with any other anti-allergic eye drops or artificial tears. However, dexamethasone sodium phosphate 0.1% eye drops (Ophthalmos, São Paulo, Brazil) q.i.d. for 10 days were permitted if an allergic crisis developed. During follow-up, crises were defined as the presence of grade 2 or 3 limbal inflammatory activity, grade 1 or 2 keratitis, and symptoms compromising daily life, or as the development of grade 3 keratitis (shield ulcer).
Outcomes
We assessed the change in severity scores for symptoms and signs during follow-up by comparison of scores between treatment groups at each follow-up point and by analyzing scores for each group separately over the study period. We also evaluated the safety and side effects of treatment by measuring visual acuity, intraocular pressures, and secondary infection and other complication rates.
Statistical analysis
Data were first analyzed descriptively, using absolute and relative frequencies for categorical variables and means and pattern-deviation for numerical variables. Age comparison between treatment groups was performed using the Student's t test for independent samples. Due to the small sample size, comparison of the distribution of VKC severity scores per treatment group were performed at each time point using Fisher's exact test. To compare the evolution of severity scores separately in each treatment group during follow-up, we used Friedman's non-parametric test. When differences were verified, comparison between follow-up periods was performed by Dunn-Bonferroni multiple comparisons to maintain the global significance level. For all statistical tests, a 5% significance level was adopted. Analyses were performed using IBM SPSS for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA), and graphics elaboration was performed using Stata 12 (StataCorp LP, TX, USA).
RESULTS
Participant characteristics
In total, 16 patients were included, with 8 per treatment group. All patients were analyzed from baseline until day 30 of follow-up, after which two patients in group 2 were excluded from further follow-up because they needed topical corticosteroids to manage allergic crises (Figure 1). There were no differences between the groups in the distribution by sex (p=0.200), age (p=0.154), or VKC subtype (p=0.151). Most patients were male (81.8%) and most presented with the limbal VKC subtype (56.3%) (Table 3).
Table 3 Homogeneity of the treatment groups
| Drug 1 | Drug 2 | Total | p | |
|---|---|---|---|---|
| Sex | 0.200 | |||
| Male | 8 (100.0%) | 5 (62.5%) | 13 (81.3%) | |
| Female | 0 (0.0%) | 3 (37.5%) | 3 (18.8%) | |
| VKC subtype | 0.151 | |||
| Tarsal | 4 (50.0%) | 1 (12.5%) | 5 (31.3%) | |
| Limbal | 4 (50.0%) | 5 (62.5%) | 9 (56.3%) | |
| Mixed | 0 (0.0%) | 2 (25.0%) | 2 (12.5%) | |
| Age (years) | 10.8 (2.4) | 12.5 (2.3) | 11.6 (2.4) | 0.154a |
The p-values are for Fisher’s exact test or Student’s t test (a). VKC = vernal keratoconjunctivitis
Differences in clinical symptoms between groups and time points
Regarding the severity scores in each group during follow-up, there were significant differences for itching (p<0.001), photophobia (p=0.006), and tearing (p<0.001) in Group 1 when comparing days 0-30-45-90. Differences were also significant for itching (p=0.020) when comparing days 0-30-45 and for foreign body sensation (p=0.047) when comparing days 0-45 in Group 2 (Figure 2, see letters a , b , and c). Differences in severity scores for itching, foreign body sensation, and photophobia were statistically significant between the treatment groups at days 90 (p=0.001), 15 (p=0.042), and 30 (p=0.041), respectively, with group 2 experiencing more severe symptoms at each point (Figure 2, see asterisks).
Differences in clinical signs between groups and time points
In group 1, the decreases in severity scores were significant when comparing days 0-30-45-90 for conjunctival hyperemia (p<0.001) and days 0-30-45 for limbal inflammatory activity (p=0.006). In Group 2, the decreases in severity scores were significant when comparing days 0-30-45 for conjunctival hyperemia (p=0.008) and when comparing days 0-45 for limbal inflammatory activity (p=0.007) (Figure 3, see letters a and b).
Differences in the severity scores for limbal inflammatory activity were statistically significant between the treatment groups at days 15 (p=0.011), 30 (p=0.007), and 45 (p=0,015). They were also significant for keratitis at day 30 (p=0.048). The decrease in severity scores for signs indicated greater improvement in Group 1 (Figure 3, see asterisks).
DISCUSSION
VKC and atopic keratoconjunctivitis are the most severe forms of ocular allergy, with visual loss a potential risk because of either the disease itself or indiscriminate corticosteroid use. In a retrospective study, Sacchetti et al.(23) observed that patients with worse VKC at presentation had more recurrences per year, more hospital visits, and worse final visual acuity. In another study, Bonini et al.(22) concluded that VKC generally has a good prognosis, with 52% of patients in their cohort showing persistent symptoms after a mean follow-up period of approximately 5 years; but, only 6% of their patients showed permanent reduction in visual acuity because of corneal damage.
In the present study, most patients in both groups had mild presentations. This may have been because they were seen in our tertiary care ophthalmic unit. Although we treat patients with more severe forms of ocular allergy, many were already being treated with tacrolimus or had already undergone supratarsal corticosteroid injection. This also limited the number of patients with diagnosed VKC who met the inclusion criteria for our study.
Tacrolimus has been shown to be effective and safe for both the control of ocular allergic crises and for the maintenance of VKC(24-26). In a randomized clinical trial, Labcharoenwongs et al.(19) compared tacrolimus 0.1% with cyclosporine 2% and reported clinical improve ment in both groups, with no significant difference between the two drugs. However, Pucci et al.(27) performed a cross-over clinical trial comparing tacrolimus 0.1% and cyclosporine 1% for the treatment of severe VKC, and they demonstrated greater improvement in objective and subjective scores in eyes treated with tacrolimus. Recently, Müller et al.(21) compared tacrolimus alone versus tacrolimus plus olopatadine for the treatment of VKC. Although clinical improvement was reported in both groups, the difference was not statistically significant, indicating the possibility of tacrolimus monotherapy for VKC. Our results support these findings, showing that tacrolimus was both effective and safe as monotherapy for VKC.
The increased numbers of CD4+ Th2 lymphocytes in the conjunctiva, together with the increased expression of costimulatory molecules and cytokines, suggest that T cells play a crucial role in the development of VKC. In addition to typical Th2-derived cytokines, Th1-type cytokines, pro-inflammatory cytokines, and a variety of chemokines, growth factors, and enzymes are overly expressed in patients with VKC(28). Tacrolimus appears to control VKC by inhibiting T-cell activation via calcineurin inhibition, thereby reducing inflammatory cytokine production (i.e., IL-4 and IL-5) and the subsequent inflammatory reactions(26).
Among the reported adverse effects of topical tacrolimus are a local burning sensation and an itching sensation, both of which tend to improve with time. Moreover, adverse symptoms are usually tolerable, with only a few reports of patients needing to stop therapy(29). Only one case of herpes simplex recurrence has been reported(18). In our study, the only adverse effect was a burning sensation in the eye, and no patient presented with major adverse events or discontinued the drug. There are no literature reports of raised intraocular pressure with tacrolimus, so it is reasonable to assume that this drug is safe for patients with glaucoma, previous steroid-induced increased intraocular pressure, or those at risk of cataract formation.
According to Attas-Fox et al.(24), blood levels of tacrolimus after topical use are clinically negligible. Ohashi et al.(18) compared different dosages of ophthalmic tacrolimus suspension (0.01%, 0.03%, and 0.1%), and concluded that the 0.1% concentration was associated with the greatest symptom improvement with the same safety profile as the other preparations. Supporting this, Ebihara et al.(30) evaluated the blood levels of tacrolimus 0.1% ointment over 12 weeks, and they concluded that the maximum blood concentration remained below 2 ng/mL, well below the level (10 ng/mL) at which the risk of systemic adverse drug reactions increases. In our experience, tacrolimus 0.03% eye drops produced good clinical outcomes, similar to the ointment formulation. Although both ointment and eye drops formulations of tacrolimus are available for ocular use in Brazil, this is only through compounding pharmacies, which limits their availability and complicates rapid access.
We chose not to include any other topical medication to be given during the follow-up period to avoid confounding factors and showed that tacrolimus could maintain all patients free from allergic crisis and improve symptoms and signs within 90 days. By contrast, two patients from group 2 were excluded from the statistical analysis after days 45 and 90 because of marked worsening of symptoms and signs that necessitated rescue therapy with topical corticosteroids. This difference in the incidence of allergic crisis was not statistically significant, and may be explained by either the small sample size, the possible differences in disease severity at baseline, or by the different efficacies of the drugs.
In the present study, strict monotherapy with either tacrolimus or sodium cromoglycate was compared. Our data indicate that tacrolimus 0.03% eye drops were superior to sodium cromoglycate 4% eye drops in alleviating the clinical symptoms (itching, foreign body sensation, and photophobia) and clinical signs (limbal inflammatory activity and keratitis) of patients with mild forms of VKC. Controlled studies with larger samples are needed to confirm our hypothesis that tacrolimus monotherapy is suitable for both acute crisis management and maintenance in VKC.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

