INTRODUCTION
Age-related macular degeneration (AMD) is a progressive degenerative disease responsible for 5% of the cases of blindness in the world(1,2). Vision impairment in AMD is characterized by choroidal neovascularization in which abnormal blood vessels that cause edema and damage are formed in the retina(3,4). Vascular endothelial growth factor (VEGF) is a potent promoter of angiogenesis, and its role in the pathogenesis of neovascular AMD is well known(5,6). Although there is no definitive treatment for the disease at present, intravitreal anti-VEGF drugs may slow its progress(7).
Bevacizumab (Avastin®; Genentech/Roche) as been used worldwide since 2005 because of its economic advantage and apparent safety and efficacy(8). Studies have demonstrated its efficacy in the treatment of AMD, and it is as effective and safe as ranibizumab (Lucentis®; Genentech/Novartis)(9)-(11). Bevacizumab is supplied as 100 mg/4 mL and 400 mg/16 mL vials but usually, only 1.25 mg/0.05 mL is used in each intravitreal injection for AMD treatment(9)-(12). Therefore, repacking into individual doses is necessary to allow proper use of the entire vial content to ensure the best economic advantage. The safety of compounding the drug as smaller doses remains under discussion. As a therapeutic protein, bevacizumab is prone to undergo physical and chemical instability reactions(13,14). Unlike other countries where bevacizumab is repacked into prefilled plastic syringes, in Brazil, the drug is repacked into ampoules and single-use glass vials. Although the stability of bevacizumab in plastic syringes has been previously studied, no information about its repackaging into glass containers is available(15)-(20). In this case, the preparation and handling procedures, in addition to the packing material, are different. Another consideration is that the ampoules are typically heat sealed, which is an additional risk of protein degradation(21). In this study, the quality and stability of bevacizumab repacked into ampoules and single-use glass vials by Brazilian compounding pharmacies were evaluated. Complementary analytical methods were used to determine changes in the physicochemical properties and biological function of bevacizumab.
METHODS
Repacked bevacizumab (3.75 mg/0.15 mL) was purchased from three compounding pharmacies (CP1, CP2, CP3). They were packed into single-use glass ampoules (CP1 and CP2) and single-use glass vials (CP3). Bevacizumab (Avastin®) from its original glass vial was used as a reference. All samples were stored at 4°C and evaluated within the shelf life defined by the suppliers. Measurements were per formed at room temperature.
To check the efficacy of the analytical methods used here, we created a positive control of bevacizumab degradation. For this purpose, bevacizumab aggregates were induced by thermal stress to mimic those that can occur during inadequate repacking, shipping, and storage of the protein solution. Thermal stress was performed by heating 0.5 mL of bevacizumab for 2 hours at 62°C.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed according to the method used in a pre vious study to detect the presence of covalent aggregates in bevacizumab samples(22). Equal amounts of bevacizumab (9 µg) were prepared in SDS sample buffer and loaded onto 7% polyacrylamide gels. The gels were run for 1.5 hours at 100 W, stained with Coomassie Blue, and destained to visualize protein bands. Nine samples from each compounding pharmacy were compared with the reference be vacizumab. Thermally stressed samples were evaluated under the same conditions for comparison.
Size-exclusion chromatography (SEC)
Size-exclusion (high-performance liquid) chromatography (SEC) was used to quantify levels of monomers and soluble aggregates of bevacizumab in the repacked thermally stressed pharmacy samples and reference samples by using a method previously developed with modifications(23). For each compounding pharmacy, 13 samples of repacked bevacizumab were analyzed in triplicate. Samples were diluted to 50 µg/mL, and 20 µL was injected into a high-resolution size-exclusion column (7.8 mm × 300 mm, 5 µm particles; Waters). Chromatography was performed by using a Shimadzu HPLC system operated at a flow rate of 1.0 mL/minute at 25 °C. Protein elution was monitored at 205 nm by using a UV-Vis absorbance detector (Shimadzu, Japan). The mobile phase and sample diluent consisted of 0.016 M Na2HPO4, 0.0014 M KH2HPO4, and 0.14 M NaCl at pH 7.4. LC solution® software (Shimadzu, Japan) was used for data acquisition and analysis. To investigate bevacizumab aggregation in samples, the relative percentage of each individual peak (monomer or high molecular weight species) to the total protein peak area was calculated(18,24). To identify monomer loss in repacked and thermally stressed samples, the area of the main peak corresponding to the IgG monomer obtained for the samples and for the reference were compared by calculating the relative monomer content(18).
Dynamic light scattering (DLS)
DLS was performed to estimate particle size distribution and to identify small-sized aggregates in the nanometer-size range. DLS measurements were made by using a ZetaSizer 3000 HSa (Malvern Instruments, UK) laser light-scattering system with a 633-nm laser source. Three samples of each compounding pharmacy were pooled from five repacked bevacizumab units for comparison with the reference. Samples were diluted to 2.63 mg/mL in 0.22-µm-filtered potassium phosphate buffer (51 mM, pH 6.2). A 2-mL aliquot of each diluted sample was added to a 1-cm path length single-use polystyrene cuvette previously rinsed with 0.22-µm-filtered purified water. All procedures were performed under laminar airflow to reduce the risk of external contamination. The Z-average diameter (Zave) and the hydrodynamic diameter distributions were acquired by using ZetaSizer Software (Malvern). Parameters were calculated by intensity analysis.
Turbidimetry
Visible protein aggregates and particles in the samples were mo nitored by measuring the absorbance at 350 nm where there is no absorbance of the known intrinsic chromophores in the protein formulation(18). For each compounding pharmacy, four samples were pooled from five repacked bevacizumab units for comparison with the drug from its original vial and thermally stressed samples. Samples were diluted to 2.5 mg/mL in 0.22-µm-filtered potassium phosphate buffer (51 mM, pH 6.2) and measured in semi-micro quartz cuvettes with a path length of 1 cm by using a UV-Vis spectrometer (Evolution 201; Thermo Scientific, USA). The mean absorbance of each sample was calculated and compared with the mean value of the reference bevacizumab.
Efficacy analysis of repacked bevacizumab
A previously described enzyme-linked immunosorbent assay (ELISA) protocol(25) was used to investigate if changes in anti-VEGF activity occurred after repacking. For each compounding pharmacy, the samples were pooled from five repacked bevacizumab units for analysis. Briefly, 100 µL of reference and repacked bevacizumab samples diluted to 250 ng/mL were mixed with 100 µL of human VEGF-165 (R&D Systems, USA) previously diluted to 500 pg/mL. PBS containing 0.2% w/v of bovine serum albumin was used as diluent for bevacizumab samples and VEGF. Next, the samples were incubated for 3 hours at room temperature under agitation. The concentration of residual-free VEGF in the bevacizumab-VEGF mixtures was measured by using a Human VEGF ELISA Kit (Quantikine, R&D Systems), according to the manufacturer's guidelines. The relative efficacy of bevacizumab was calculated by subtracting the residual-free levels of VEGF from the initial concentration (250 pg/mL after mixture). For each sample, two runs were performed, and the sample was repeated in duplicate in each analysis.
Statistical analysis
The results are presented as the mean ± SD. Data were tested by using the D'Agostino-Pearson normality test and analyzed by using Student's t-test and ANOVA. GraphPad Prism software, v.5.04 (GraphPad Software Inc., USA) was used for statistical analysis. Statistical significance was defined as p<0.05.
RESULTS
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Since protein electrophoretic mobility in SDS-PAGE depends on its molecular weight (MW), it is possible to separate and identify proteins in monomeric and aggregated forms. Reference bevacizumab showed a major band that represents IgG monomers, a higher MW band that corresponds to covalent aggregates, and lower MW bands with low intensity that may represent protein fragments (Figure 1). Similar bands for reference and repacked bevacizumab were obtained while thermally stressed samples showed two extra bands of higher MW (Figure 1). These results clearly demonstrated that, under the established conditions, the method could detect aggregation in the samples. The lack of bands at the top of the repacked bevacizumab sample lanes suggests the absence of covalent aggregates.
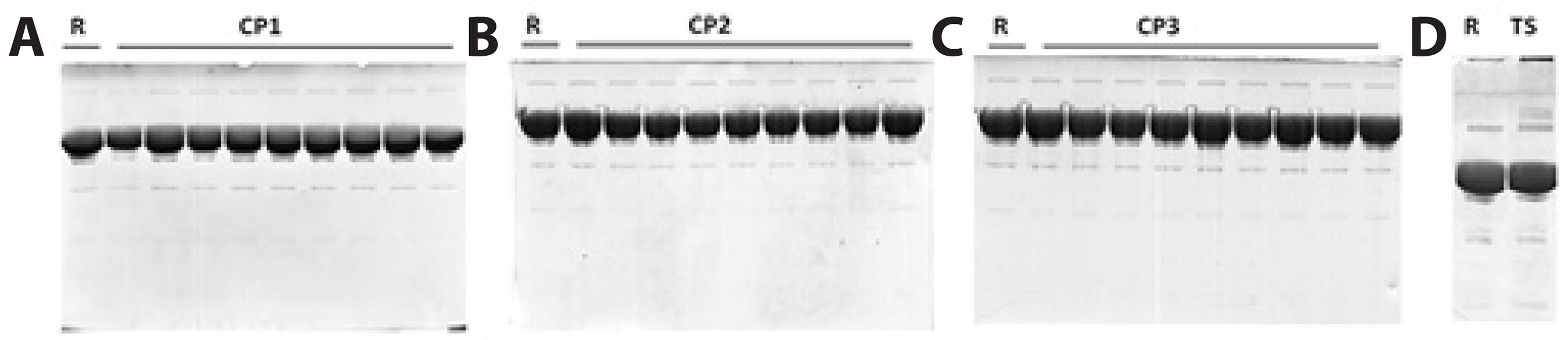
Figure 1 SDS-PAGE of bevacizumab repacked by CP1 (A), CP2 (B), and CP3 (C). Reference bevacizumab was run in each gel for comparison. Thermally stressed samples showed two extra bands of higher molecular weight that were not observed in the reference or repacked bevacizumab (D). R= reference bevacizumab; CP= compounding pharmacy; TS= thermally stressed bevacizumab.
SEC
SEC analysis is a classical method for detection of potential frag mentation and aggregation of proteins(26). SEC results are reported in table 1. Chromatograms obtained for reference bevacizumab de monstrated a major peak eluting at a volume expected for mo nomeric IgG when analyzed under these conditions, and two relatively minor peaks with shorter retention times representing soluble protein oligomers were observed (Figure 2). No significant changes in the chromatograms were observed between the repacked bevacizumab samples and the reference in either the highest or lowest molecular weight regions (Figure 2). From the SEC-UV signal at 205 nm, the relative amount of monomer and aggregates in the total protein peak area was calculated (Table 1). A small increase in the first species of high molecular weight aggregates (HMW1) was observed for the three compounding pharmacies relative to that in the reference bevacizumab (CP1, p<0.01; CP2, p<0.0001; and CP3, p=0.0001). A slight increase in the second species of high molecular weight aggregates (HMW2) was observed for CP1 and CP2 (p<0.05). These increased percentages of HMW aggregates were accompanied by a reduction in bevacizumab monomers observed for the three compounding pharmacies (CP1, p<0.05; CP2, p<0.001; and CP3, p<0.01). To investigate monomer loss, relative monomer content was calculated for the monomer peak areas in the repacked and reference bevacizumab (considered as 100%) samples (Table 1). Monomer levels in samples from all compounding pharmacies were similar to those in the reference (relative monomer content of 103% ± 3%, 99.2% ± 3.4%, and 99.5% ± 4.3%, respectively, for CP1, CP2, and CP3). Monomer loss ranged from -8.4% to 2.2%, -3.5% to 5.0%, and -5.4% to 7.5%, respectively, for CP1, CP2, and CP3. The mean monomer loss in the samples from the three compounding pharmacies was <1%. The thermally degraded samples of bevacizumab were also analyzed, and the chromatograms showed increased percentages of soluble aggregates (15.0% ± 3.4% of HMW1 and 2.5% ± 1.6% of HMW2) relative to those in the reference sample (2.14% ± 0.21% of HMW1 and 0.0% ± 0% of HMW2), which clearly indicated aggregation. The chromatograms also showed a reduced percentage of monomeric bevacizumab (83.33% ± 5.48%) relative to that in the reference sample (97.85% ± 0.21%). Drastic monomer loss (36.11% ± 7.14%) was detected when the monomer peak areas of the thermally stressed samples and reference samples (considered as 100%) were compared. These data validate the stability-indicating capability of the method, which ensures that, if presented in repacked samples, protein aggregation and monomer loss would be detected.
Table 1 Relative amounts of monomers and aggregates of repacked bevacizumab
| Sample | Monomers (%) | HMW1 (%) | HMW2 (%) | Relative monomer content (%) |
|---|---|---|---|---|
| Reference | 97.78 ± 0.11*** | 02.02 ± 0.08 | 0.19 ± 0.03* | 100.00 ± 1.90 |
| CP1 | 97.68 ± 0.09*** | 02.14 ± 0.10** | 0.18 ± 0.01* | 103.00 ± 3.00 |
| Reference | 97.69 ± 0.09*** | 02.12 ± 0.05* | 0.21 ± 0.01* | 100.00 ± 4.70 |
| CP2 | 97.56 ± 0.06*** | 02.24 ± 0.06**** | 0.20 ± 0.02* | 099.20 ± 3.40 |
| Reference | 97.86 ± 0.09*** | 01.98 ± 0.08 | 0.15 ± 0.02* | 100.00 ± 1.70 |
| CP3 | 97.71 ± 0.09*** | 02.14 ± 0.08**** | 0.14 ± 0.03* | 099.50 ± 4.30 |
| Reference | 97.85 ± 0.21*** | 02.15 ± 0.21* | 0.00 ± 0.00* | 100.00 ± 1.80 |
| Thermal stressed | 82.48 ± 5.00*** | 15.00 ± 3.4 | 2.50 ± 1.60* | 065.90 ± 0.40 |
HMW= high molecular weight aggregates; CP= compounding pharmacy. Values given as mean ± SD.
*p<0.05;
**p<0.01;
***p<0.001;
****p<0.0001 compared with the corresponding bevacizumab reference. Data were statistically analyzed by unpaired Student’s t test. Monomers (%) = Main peak area/total peak area × 100; HMW1 (%) = HMW1 peak area/total peak area × 100; HMW2 (%) = HMW2 peak area/total peak area × 100. Relative monomer content (%) = Sample main peak area/reference main peak area.
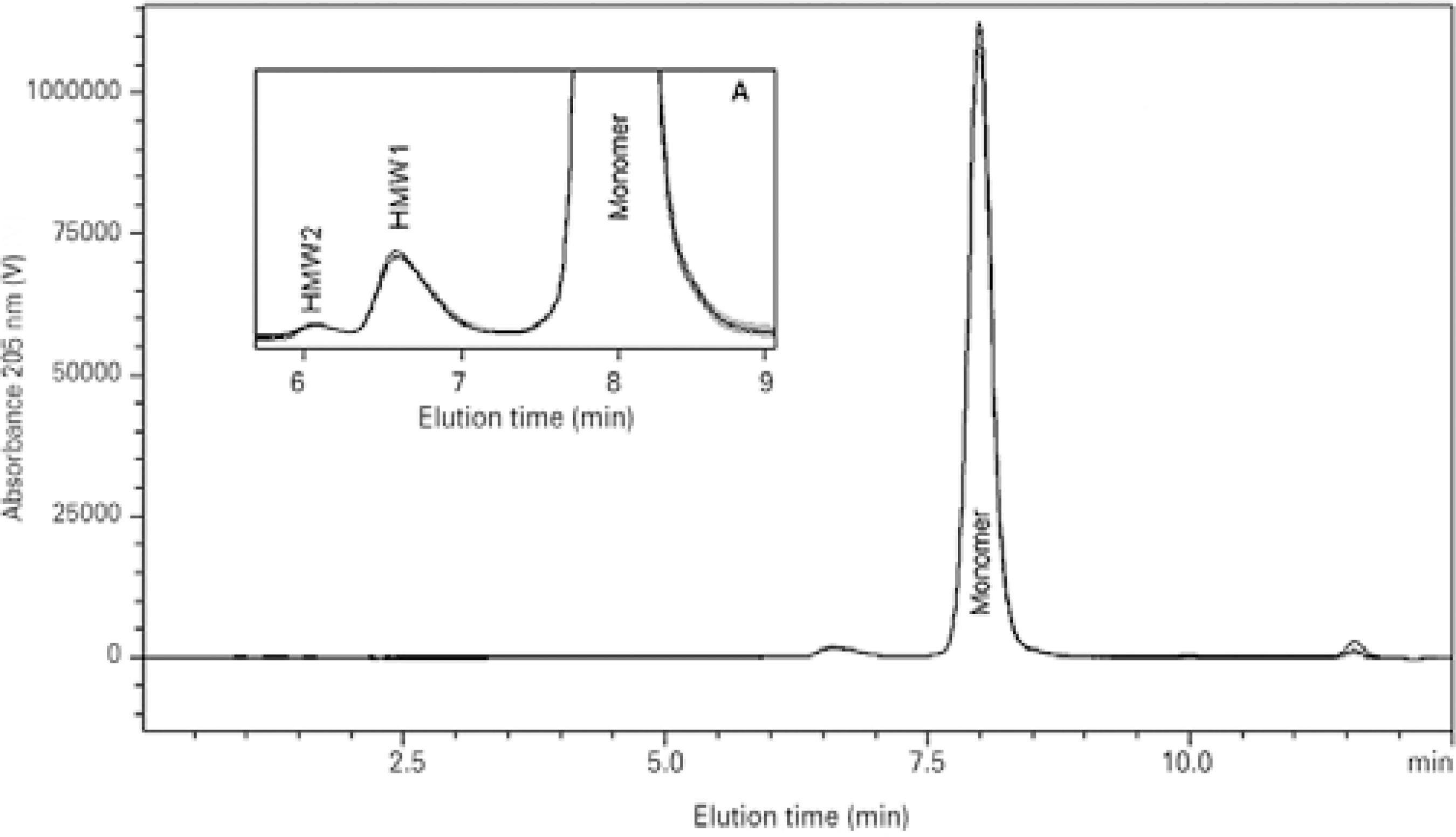
Figure 2 Overlapped size-exclusion (high-performance liquid) chromatography (SEC) chromatograms of reference bevacizumab and bevacizumab repacked in ampoules and single-use glass vials by three external compounding pharmacies (CP1, CP2, and CP3). Details of the 6-9 min region showing high molecular weight species (HMW) peaks, Inset A.
DLS
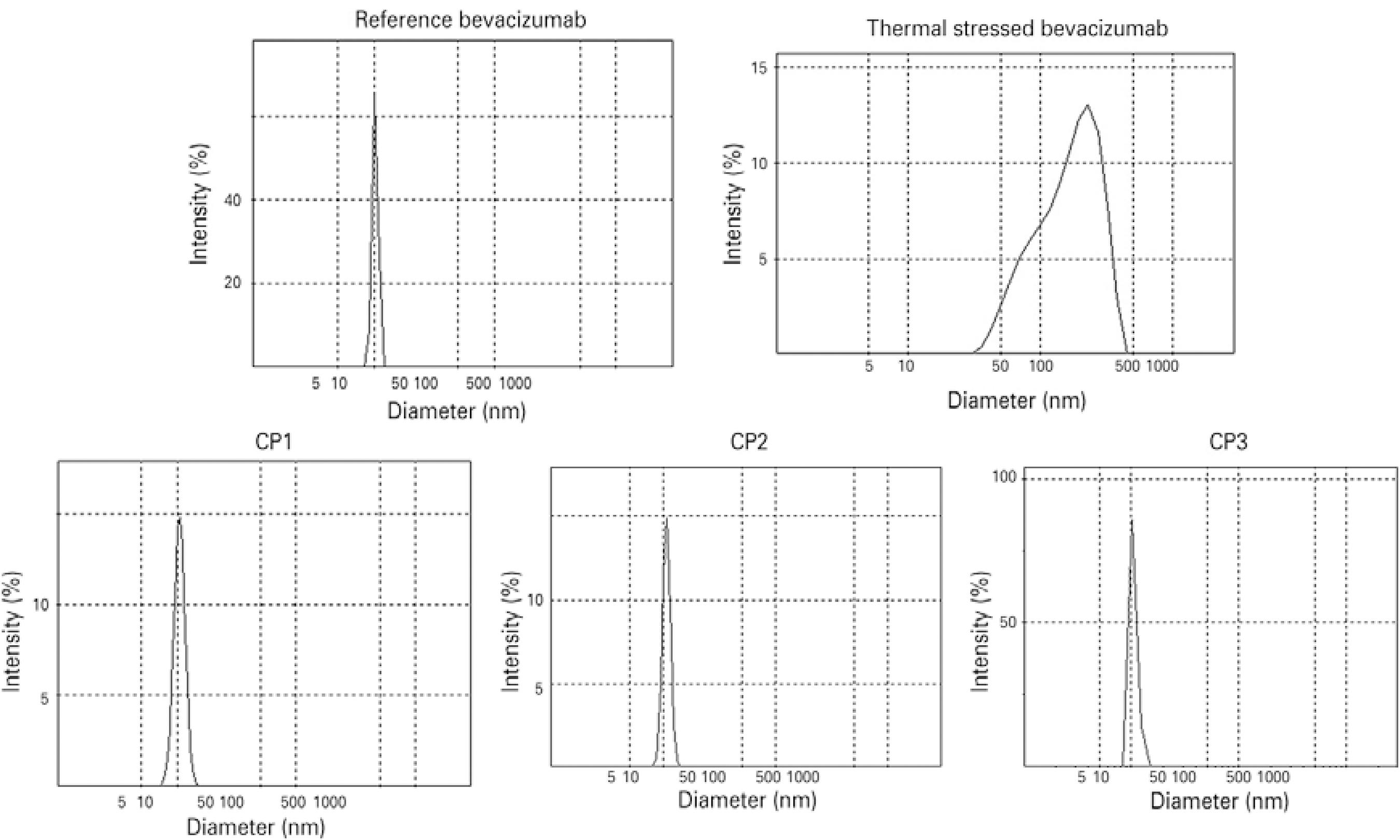
DLS was used to investigate small-sized aggregates in the nanometer-size range. Results for the reference bevacizumab showed an average hydrodynamic diameter of 12.16 ± 1.77 nm and a single peak of 12.33 ± 2.10 nm, which correspond to IgG monomers and dimers according to previous studies (Figure 3)(18). Comparable results were obtained for repacked samples with no extra peak registered (Figure 3). The CP1, CP2, and CP3 samples showed average hydrodynamic diameters of 10.44 ± 0.04 nm, 10.76 ± 0.08 nm, and 11.09 ± 0.25 nm, respectively. The thermally stressed samples had DLS peaks shifted to larger sizes, with an average hydrodynamic diameter of 128.8 nm, which is indicative of protein aggregation (Figure 3). Overall, the DLS results showed no evidence of small-sized aggregates in the reference or repacked samples.
Turbidimetry
The absorbance (AU) values at 350 nm were 0.010 ± 0.002, 0.014 ± 0.007, 0.011 ± 0.006, and 0.011 ± 0.009 for the reference bevacizumab and repacked bevacizumab samples CP1, CP2, and CP3, respectively. There were no statistically significant differences in optical density between the repacked and reference samples. The turbidity of the thermally stressed bevacizumab was 0.078 ± 0.010 AU at 350 nm, which was an approximately 8-fold increase relative to the value of the reference sample.
Efficacy analysis of repacked bevacizumab
The efficacies of the repacked bevacizumab samples were analyzed by ELISA to determine if the bevacizumab VEGF binding capacity was affected after repackage. The results are summarized in table 2. After 3 hours of reaction, the amounts of the initial VEGF that reacted in the reference bevacizumab, CP1, CP2, and CP3 samples were, 84.40% ± 2.12%, 83.02% ± 5.08%, 83.38% ± 7.62%, and 83.55% ± 5.85%, respectively, which showed that the VEGF binding capacities were similar. Setting the binding capacity of reference bevacizumab as 100%, the relative efficacies obtained were 97.46% ± 4.72%, 98.71% ± 6.58%, and 98.07% ± 5.47%, respectively, for the CP1, CP2, and CP3 samples, which showed that there was <3% loss of VEGF binding capacity after repacking in all three samples.
Table 2 VEGF binding capacity of repacked bevacizumab
| Sample | Initial VEGF | Inicial BVZ | Final VEGF | Consumed | Consumed | Relative efficacy |
|---|---|---|---|---|---|---|
| (pg/mL) | (ng/mL) | (pg/mL) | VEGF (pg/mL) | VEGF (%) | of bevacizumab (%) | |
| A | B | C | D = A-C | E = D / 250 | - | |
| Reference | 250 | 125 | 39.00 ± 05.26 | 211.00 ± 05.25 | 84.40 ± 2.12 | 100 |
| CP1 | 250 | 125 | 42.46 ± 12.71 | 207.54 ± 12.71 | 83.02 ± 5.08 | 97.46 ± 4.72 |
| CP2 | 250 | 125 | 41.55 ± 19.06 | 208.45 ± 19.06 | 83.38 ± 7.62 | 98.71 ± 6.58 |
| CP3 | 250 | 125 | 41.12 ± 14.62 | 208.88 ± 14.62 | 83.55 ± 5.85 | 98.07 ± 5.47 |
BVZ= bevacizumab; CP= compounding pharmacy. The binding capacity of reference bevacizumab was set as 100%. Values are given as mean ± SD.
DISCUSSION
In this study, the stability and efficacy of repacked bevacizumab from Brazilian compounding pharmacies were assessed and compared with bevacizumab from its original glass vial. The methods were selected to detect changes in VEGF binding capacity and formation of aggregates of various sizes(27). Analysis of the efficacy by SEC, SDS-PAGE, DLS, and turbidimetry of the thermally degraded samples showed that bevacizumab had good physical instability; the main observed effect was the occurrence of aggregation.
Because of the particular structure of proteins, assessment of biological activity during stability studies is convenient as a complementary test to physicochemical analysis(14). The anti-VEGF activity of bevacizumab samples was investigated by using ELISA, and the results showed that the biological function of bevacizumab was pre served after repacking, with <3% loss of VEGF binding capacity relative to that of the reference. Previous studies have shown that bevacizumab maintained its biological stability after repackaging in syringes. One study demonstrated similar anti-VEGF activity between the reference and repacked bevacizumab stored ≤3 months at 4°C and ≤7 days at room temperature, even when exposed to indirect light sources(20). The other studies showed degradation of 1.6%, 8.8%, and 15.9% of repacked bevacizumab stored at 4°C after 1 week, 3 months, and 6 months, respectively(15).
In the present study, SEC was used to quantify bevacizumab mo nomers and soluble aggregates in the samples. The relative mono mer content in the repacked samples was investigated because a decrease in monomeric bevacizumab can be related to protein aggregation, fragmentation, or other instabilities, as confirmed by ana lyzing thermally stressed bevacizumab samples(28,29). We found that the monomer loss was ≤1% for the repacked samples relative to that of the reference bevacizumab. When the relative percentage of each individual peak (monomer or high molecular weight species) in the total protein peak area was calculated, a slight increase in bevacizumab aggregates was observed in the three compounding pharmacy samples together with a decrease in the bevacizumab monomers. SEC has been previously used to investigate bevacizumab stability after repacking(16)-(19). Palmer and colleagues reported no differences in the levels of bevacizumab monomers or protein aggregates between the samples repacked in syringes and reference bevacizumab(19). Paul and colleagues found no differences between the relative percentages of monomer, dimer, and high molecular weight species of the total peak area of reference bevacizumab chromatograms after storage for 30 and 90 days in polypropylene syringes(18). However, Liu and colleagues found substantial monomer loss in bevacizumab repacked in plastic syringes in two of four external pharmacies studied(17).
Covalent aggregates, usually formed by disulfide bonds, can occur after protein unfolding, which normally results in the exposure of hydrophobic regions and eventually, in the case of IgG, of free cysteins. SDS-PAGE is specific for detection of covalent aggregates because non-covalent aggregates dissociate under the denaturing conditions caused by the presence of SDS(28,30). The SDS-PAGE results showed that repacking did not lead to the formation of covalent IgG aggregates in bevacizumab samples. Compared with the reference, the repacked samples showed no extra bands or differences in the intensities of the bands between the gel lanes. The results obtained for thermally stressed bevacizumab indicated that if covalent aggregates were present in the samples, they were visualized as well-resolved bands of higher molecular weight. Previous studies have also reported similar SDS-PAGE results for reference and bevacizumab syringes supplied by external compounding pharmacies(16,19).
DLS was performed to investigate changes in the mean hydrodynamic diameter of proteins in the bevacizumab samples as a result of nano-sized aggregates(29). The results showed no evidence of small-sized aggregates in the reference or repacked samples; both showed a single peak by DLS and equivalent mean hydrodynamic diameters. The thermally stressed bevacizumab results showed peak shifts indicative of larger sizes and higher average hydrodynamic diameter, which was indicative of protein aggregation.
Turbidimetry was used to monitor protein aggregation by measuring the optical density of samples on the basis of light scattering in the near UV region, where proteins have insignificant absorption(31). We found equivalent optical densities between the three compounding pharmacy repacked samples and reference samples, which in dicated that there were no visible protein aggregates or particles. Similar results have been previously obtained by DLS and turbidimetric analysis of bevacizumab repacked in plastic syringes, which showed no changes in hydrodynamic diameter or optical density of samples stored for 3 months(18).
Medical community warnings about the quality of bevacizumab after repackaging have stimulated several studies on its sta bility in single-use plastic syringes. These studies examined stability after repacking in research laboratories, which was important for un derstanding the drug's behavior, as well as the stability of repacked bevacizumab purchased from external pharmacies, which provided a better understanding of the quality of clinically used drugs. The main instability observed in previous studies was caused by contamination with silicone oil from syringes(17)-(19). Although we did not perform a par ticle characterization analysis, we believe that repacking in glass containers might avoid particulate contamination and should be considered as an option. Because repacked bevacizumab is used clinically worldwide, we support the idea that the quality of bevacizumab repacked in different countries or regions should be investigated. Despite the relevance of studying repacking by external pharmacies, only a few studies have been published so far. Our results suggest that bevacizumab repacked in glass containers for intravitreal administration is physically, chemically, and biologically stable.
CONCLUSION
In this study, the quality and stability of bevacizumab repacked into ampoules and single-use glass vials by Brazilian compounding pharmacies were assessed. Complementary analytical methods were used to determine changes in the physicochemical properties and biological functions of bevacizumab. The analyzed samples of repacked bevacizumab showed no significant aggregation, fragmentation, or loss of biological activity. Therefore, this study suggests that if precautions are taken, the formulation remains stable after compounding in ampoules and single-use glass vials.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

