INTRODUCTION
Retinal vein occlusion (RVO) is an important cause of visual loss worldwide. It is the second-most common retinal vascular disorder, and epidemiologic studies reported prevalence rates of 0.7-1.6% in the general population(1-2). An estimated 520 new cases per 1 million people develop annually(3) and 15.3% of cases involve the central retinal vein.
Macular edema, ischemic maculopathy, anterior and posterior segment neovascularization, vitreous hemorrhage, and neovascular glaucoma (NVG) are possible complications associated with central retinal vein occlusion (CRVO). The anterior segment is the main site of neovascularization in CRVO. The risk of development increases with the degree of retinal ischemia, and it is most likely to develop during the first 3 months after occlusion(4,5). The cumulative incidence of NVG in ischemic CRVO is approximately 40% over 1 year, compared with 10% in nonischemic eyes(6).
The Central Vein Occlusion Study (CVOS) reported that scatter panretinal laser photocoagulation (PRP) is recommended promptly after the development of neovascularization over 2 h or more in the iris or any angle neovascularization(7).
Recent prospective, randomized, controlled trials evaluated intravitreally injected drugs for treating CRVO and tried to define treatment strategies for macular edema secondary to CRVO.
Steroids reduce vascular permeability and stabilize the blood-re tina barrier(8). The mechanism involves inhibition of inflammatory mediators and vascular permeability factors such as vascular endothelial growth factor (VEGF); thus, it may prevent neovascularization(9,10). The SCORE study compared the efficacy and safety of two doses (1 and 4 mg) of intravitreal triamcinolone acetonide (IVTA) for the treatment of macular edema after CRVO in 272 eyes(11). The study reported improved BCVA in 27% of eyes treated with 1 mg of IVTA and fewer ocular adverse events in this group. Neovascularization occurred in 9.8% of eyes treated with 1 mg of IVTA and 4.4% in those treated at a dose of 4 mg. Implantation of sustained corticosteroid delivery devices resulted in improved best-corrected visual acuity (BCVA) in other studies(12).
VEGF plays a key role in the pathophysiology of CRVO and its complications. Several studies proposed treatment with the anti-VEGF drugs bevacizumab (Avastin, Genentech Inc., South San Francisco, CA), ranibizumab (Lucentis, Genentech Inc.), and aflibercept (Eylea, Regeneron Pharmaceuticals, Tarrytown, NY).
The CRUISE study illustrated that patients treated with monthly intravitreal ranibizumab (0.3 or 0.5 mg) achieved better results than controls(13). The improved BCVA was maintained at the 12-month endpoint.
The HORIZON trial followed the same patients who enrolled in the CRUISE study during the second year. At the end of 24 months, BCVA did not differ significantly among the three groups (0.3 mg, 0.5 mg, and sham)(14).
Bevacizumab is an anti-VEGF drug used to manage retinal vascular disorders such as age-related macular degeneration, diabetic ede ma, and retinal vein occlusions(15-18). Several retrospective and prospective studies reported decreased retinal thickness and improved BCVA after intravitreal injections of the drug(19,20).
Although most recent studies suggested the therapeutic benefits of intravitreal steroids and anti-VEGF for treating macular edema secondary to CRVO, none focused on the effects of such treatments for preventing anterior segment neovascularization (ASN) and NVG as a primary endpoint.
The purpose of the this prospective study was to analyze the effects of intravitreal bevacizumab (IVB) injections compared with IVTA or sham injections for preventing ASN and NVG in patients with macular edema due to CRVO.
METHODS
Study design
This 12-month randomized, double-masked, sham-controlled study was designed to evaluate the incidence rates of ASN and NVG in three groups treated for macular edema secondary to CRVO.
The study included a 28-day screening period, a 6-month treatment period (baseline to month 6) in which patients received monthly injections, and an additional 6-month, open-label PRN treatment period (month 6 to final study visit).
The study was conducted according to the tenets of the Declaration of Helsinki and federal laws. All patients were informed about the purpose of the study, and they provided informed consent. The ethics committee of our institution approved the study. The primary outcome was the presence of ASN in the study eye, as determined by ophthalmologic examination at the 6-month follow-up visit.
Screening and eligibility
The primary investigator (LFAL) determined patient eligibility at the Retina Division of the Department of Ophthalmology, Federal University of São Paulo, using the criteria in table 1.
Table 1 Inclusion and exclusion criteria
| Inclusion criteria* |
| ≥18 years of age with macular edema secondary to CRVO and less than 90 days since symptoms appeared |
| BCVA ≤20/40 according to the Snellen chart |
| Central foveal thickness ≥250 µm according to a central 1-mm diameter circle with a Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) |
| Exclusion criteria* |
| Any iris or angle neovascularization evident on slit lamp or gonioscopy examination without pupillary dilation |
| Presence of macular edema due to a cause other than CRVO |
| Prior episode of RVO |
| IOP ≥25 mmHg, open-angle glaucoma (either primary open-angle glaucoma or other cause), prior steroid-induced IOP elevation, or pseudoexfoliation |
| Evidence on examination of any diabetic retinopathy |
| History or presence of wet or dry AMD |
| Any previous treatment for macular edema |
| Previous panretinal scatter photocoagulation or sector laser photocoagulation |
| Prior anti-VEGF treatment |
| Any ocular surgery within 6 months before baseline |
| Prior pars plana vitrectomy |
| Intra or periocular acute infection |
*pertains to the study eye, except where noted otherwise.
CRVO= central retinal vein occlusion; BCVA= best-corrected visual acuity; BRVO= branch retinal vein occlusion; RVO= retinal vein occlusion; IOP= intraocular pressure; AMD= age related macular degeneration; VEGF= vascular endothelial growth factor. CRVO was defined as an eye that had retinal hemorrhage or other biomicroscopic evidence of RVO (e.g., telangiectatic capillary bed) and a dilated (or previously dilated) venous system in ≥3 quadrants of the retina drained by the affected vein.
During the screening visit, after providing informed consent, all participants provided a complete medical history and underwent an ophthalmologic examination that included measurements of BCVA using a Snellen chart, slit-lamp examination, gonioscopy, measurement of intraocular pressure (IOP), pupillary reflex, binocular fun dus examination, optical coherence tomography (SD-OCT) (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany), and wide-angle fluorescein angiography (FA) (HRA, Heidelberg Engineering).
Randomization
If the physician investigator judged a patient eligible for participation in the study, then he or she was randomized to one of three treatment groups as follows Figure 1: group 1, sham injections; group 2, 1.25-mg IVB injections; and group 3, 1-mg IVTA injections.
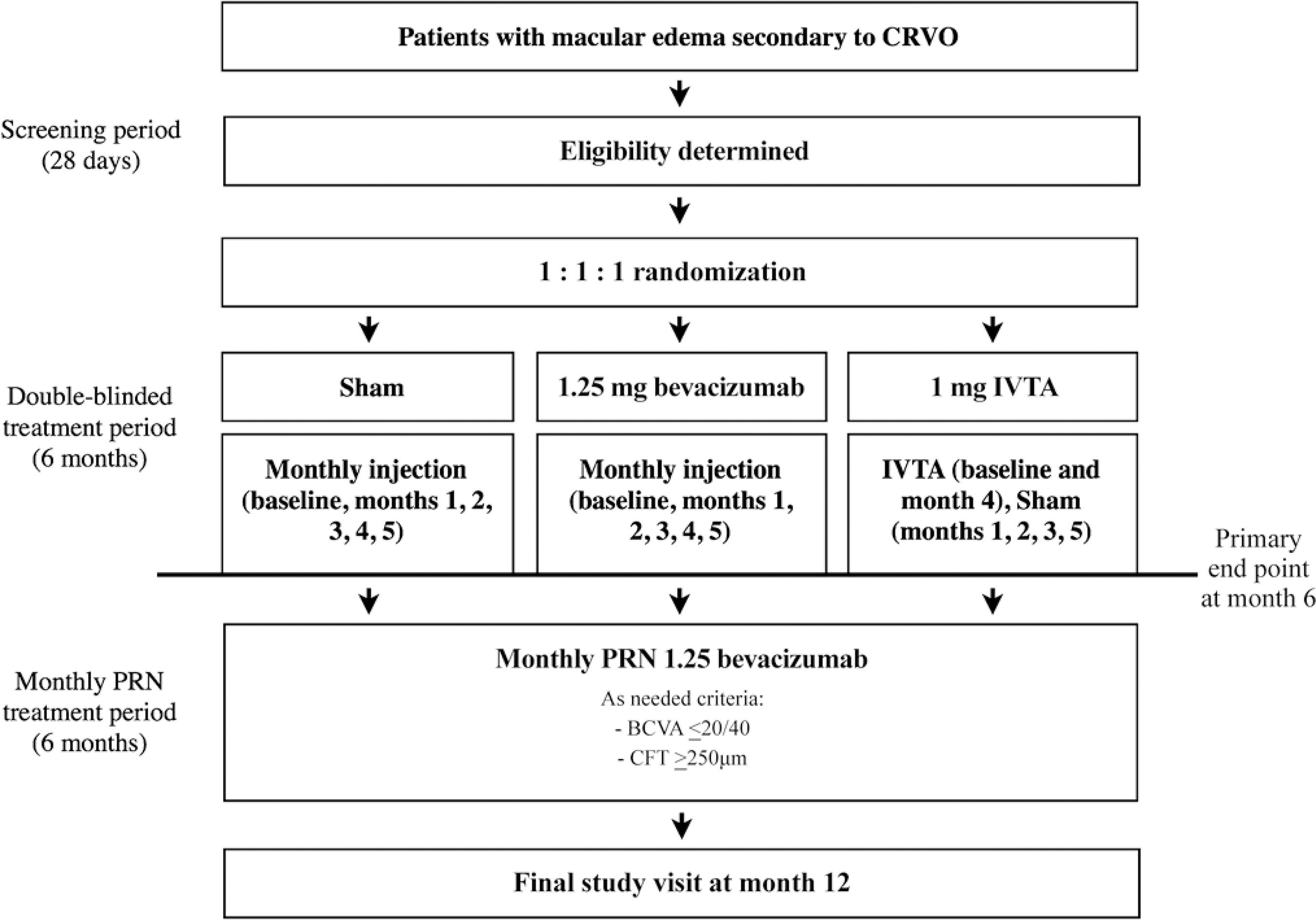
Figure 1. Study design. Eligible patients were randomized 1:1:1 to receive monthly sham, 1.25-mg intravitreal bevacizumab (IVB), or 1-mg intravitreal triamcinolone acetonide (IVTA) injections during the 6-month treatment period. During the monthly pro ra nata (PRN) treatment observation period, patients were eligible to receive monthly intraocular 1.25-mg IVB injections if they had a Snellen equivalent best-corrected visual acuity of 20/40 or worse according to the Snellen chart or central foveal thickness of 250 µm or more according based on spectral-domain optical coherence tomography.CRVO=central retinal vein occlusion.
The patients in groups 1 and 2 received monthly sham and 1.25-mg IVB injections, respectively, at baseline and months 1, 2, 3, 4, and 5. The patients in group 3 received IVTA injections at baseline and month 4; at months 1, 2, 3, and 5, the eyes of patients randomized to group 3 received sham injections. Moreover, for patient ran domization, a computer-generated randomization table was created (Stata v11, StataCorp, College Station, TX). Participants were randomized 1:1:1 to treatment groups with block sizes of three and six. An investigator not otherwise involved in the trial performed all randomization processes.
One eye of each patient was included in the study. If both eyes were eligible, the eye with the worse BCVA at screening was selected. Patients and evaluating physicians were masked to treatment during the first 6 months of the study. The physician who administered the injections (LFAL) did not perform examinations or outcome assessments, and he had knowledge about the drug administered or sham injection at the time of injection.
Study visits and assessments
During the 6-month follow-up period, study visits occurred on day 0 (baseline) and months 1, 2, 3, 4, 5, and 6. During the monthly PRN treatment period, patients were eligible to receive monthly 1.25-mg IVB injections if they had BCVA in the study eye of 20/40 or worse according to the Snellen chart and/or CFT of 250 µm or more according to SD-OCT. The patients continued monthly follow-up (months 7, 8, 9, 10, and 11 and the final study visit). At each visit, the recorded patient data included BCVA measured using a Snellen chart, slit-lamp examination, and gonioscopy; IOP measured via Goldmann tonometry, binocular fundus examination, and SD-OCT assessment of CFT. Wide-angle FA was performed at baseline and visits 6 and 12.
Eyes with clinical findings of retinal ischemia (VA<20/200, relative afferent pupillary defect [APD], and cotton wool spots) were evaluated and correlated with the development of ASN.
The FA findings were classified as ischemic when more than 10 disc areas of retinal capillary nonperfusion were present and perfused (nonischemic) while fewer than 10 disc areas of nonperfusion were present(5). The perfusion status of FA was considered indeterminate when intraretinal hemorrhage prevented visualization of fluorescein in the retinal capillaries during the experiment.
At each visit, the patients provided a medical history, the medication was reviewed, and safety was assessed. Any new sign, symptom, illness, or worsening of any preexisting medical condition was recorded as an adverse event (AE). An AE was considered as a serious AE (SAE) when it resulted in death or when it was life-threatening, it required prolonged hospitalization, it caused persistent or significant disability, it was a congenital anomaly/birth defect, or it was considered a significant medical event by the investigator. Furthermore, patients who discontinued the study before the month 12 visit were encouraged to return for an early final study visit 30 days after their last injection or analysis. If ASN was detected at any time in the study, the patient was referred for scatter PRP according to recent recommendations. If NVG was detected despite PRP, patients were referred to the glaucoma sector for follow-up and treatment.
Intraocular injections
The procedure for drug administration at the ophthalmic surgical center of the Federal University of São Paulo was as described further. Topical anesthetic drops were administered, and a lid speculum was used. A 5% povidone iodine drop was instilled as prophylaxis against infection 5 min before the procedure. A 30-gauge needle was inserted through the pars plana, and 0.05 ml of bevacizumab (OPHTHALMOS® 25 mg/ml São Paulo, Brazil) or 0.025 ml of triamci nolone acetonide (OPHTAAC® 40 mg/ml OPHTHALMOS São Paulo, Brazil) was injected(13,21). The procedure for administering sham injections was similar to that for the IVB and IVTA injections, except that the hub of a syringe without a needle was placed against the injection site and the syringe plunger was depressed to mimic an injection. The ability to count fingers with the study eye was assessed 1 min after the injection. No topical antibiotics were prescribed postoperatively for any patient. An additional visit within 5 days after each injection was scheduled as a postoperative evaluation.
Outcome measures
The primary outcome measure was the incidence of ASN at month 6. The secondary outcomes included the mean changes from baseline in BCVA and CFT over time to month 12. The safety outcomes included the incidence and severity of ocular, nonocular, and systemic AEs.
Statistical analysis
Data were analyzed and expressed as means and standard deviations or frequencies (%). Comparisons of continuous and categorical variables among the treatment groups were performed using the Kruskal-Wallis test and Fisher's exact test, respectively. Post-hoc analyses were performed using the Bonferroni test. p<0.05 was considered statistically significant. All analyses were performed using Stata v11.
RESULTS
Baseline demographics and ocular characteristic.
Between September 2013 and May 2015, 35 eyes of 35 patients in the Retina Sector of the Federal University of São Paulo, Brazil, were randomized, that is, 10, 14, and 11 eyes to the sham, IVB, and IVTA groups, respectively. Thirteen patients completed the screening visit, but they were excluded as follows: seven had glaucoma; three did not provide informed consent; two were excluded because of social issues; and one was excluded because ASN was detected during the screening visit. The patient demographic data were similar across the treatment groups Table 2. The baseline ocular characteristics were also similar across treatment groups excluding APD (p<0.05).
Table 2 Patient demographics and baseline ocular characteristics
| Sham (n=10) | Bevacizumab 1.25 mg (n=14) | Triamcinolone acetonide 1 mg (n=11) | Among-group p value* | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 55.6 (14.41) | 61.86 (12.62) | 60 (12.63) | 0.450 |
| Range | 31-83 | 37-89 | 45-80 | |
| Gender, n (%) | 1.000 | |||
| Male | 6 (60%) | 9 (64.29%) | 7 (63.64%) | |
| Female | 4 (40%) | 5 (35.71%) | 4 (36.36%) | |
| Race, **n (%) | 0.460 | |||
| White | 6 (60%) | 7 (50.00%) | 9 (81.82%) | |
| Black | 2 (20%) | 4 (28.57%) | 2 (18.18%) | |
| Asiatic | 1 (10%) | 0 | 0 | |
| Other | 1 (10%) | 3 (21.43%) | 0 | |
| Time of symptoms | 0.482 | |||
| Mean (SD) | 31.9 (18.60) | 25.42 (22.07) | 37.54 (30.11) | |
| Range | 4-60 | 3-60 | 3-85 | |
| BCVA (logMAR) | 0.275 | |||
| Mean (SD) | 1.64 (0.44) | 1.32 (0.53) | 1.40 (0.62) | |
| Range | 0.9-2.2 | 0.3-1.79 | 0.5-2.2 | |
| VA<20/200, n (%) | 9 (90%) | 11 (78.57%) | 7 (63.64%) | 0.418 |
| Cotton wool spots, n (%) | 6 (60%) | 08 (57.14%) | 7 (63.64%) | 1.000 |
| Afferent pupillary defect, n (%) | 6 (60%) | 07 (50.00%) | 2 (20.00%) | 0.029 |
| Lens status, n (%) | 1.000 | |||
| Phakic | 10 (100%) | 13 (92.86%) | 11 (100%) | |
| Pseudophakic | 0 | 01 (07.14%) | 0 | |
| >10 DA of capillary non-perfusion, n (%) | 0.357 | |||
| Yes | 5 (55.56%) | 3 (21.43%) | 4 (36.36%) | |
| No | 3 (33.33%) | 4 (28.57%) | 4 (36.36%) | |
| Undetermined | 1 (11.11%) | 7 (50.00%) | 3 (27.27%) | |
| CFT (SD-OCT), µm | 0.500 | |||
| Mean (SD) | 774.87 (276.85) | 706 (261.93) | 813 (152.90) | |
| Range | 252-1059 | 262-1146 | 555-999 |
*P-values less than 0.05 were considered statistically significant;
**multiracial patients were counted in each race category that they indicated. SD=standard deviation; BCVA=best correct visual acuity; VA=visual acuity; CFT=central foveal thickness; SD-OCT=spectral domain optical coherence tomography.
The mean patient age was 59.48 years (range, 31-89 years), and 60% of patients were men. The average time for symptom development was 31.08 days (range, 3-85 days). The mean baseline BCVA (logarithm of the minimum angle of resolution [logMAR]) of the study eye was 1.43 (±0.53) (Snellen equivalent, 20/538), and 27 eyes (77.14%) had BCVA of less than 20/200.
The baseline biomicroscopic examination illustrated that 34 (97.14%) eyes were phakic, and one (2.86%) was pseudophakic. Fifteen (42.86%) eyes had an APD. The baseline funduscopic examination indicated that 21 (60%) eyes had cotton-wool spots, and the mean CFT was 754.51 µm (range, 252-1146 µm).
More than 10 disc areas of retinal capillary nonperfusion were present on the baseline FA images in 12 (34.29%) eyes. Five, four, and three of these eyes were randomized to sham, IVTA, and IVB treatment, respectively. The other 11 eyes had less than 10 disc areas of retinal capillary nonperfusion, and in 11 eyes, the area of nonperfusion was undetermined (p=0.357).
Primary endpoint
Eight (22.86%) eyes had ASN. Five eyes randomized to sham treatment (50%) and three eyes randomized to 1 mg IVTA (27.27%) developed ASN. No eyes randomized to IVB developed ASN in the iris and/or angle during 12 months of follow-up (p=0.009).
The overall mean time for development of ASN was 59.75 ± 42.79 days, that is, 65.6 ± 54.22 days in the sham group and 50 ± 17.32 days in the IVTA group (p>0.05).
We also analyzed the presence of clinically diagnosed retinal ischemia (BCVA<20/200, APD, and cotton-wool spots). At baseline, 10 eyes presented clinical findings suggestive of retinal ischemia. Of these, five were randomized to the IVB group, and none developed ASN during the follow-up period. The remaining five eyes with ischemia developed ASN, three and two of which were randomized to the sham (p=0.17) and IVTA groups (p=0.056). Two patients randomized to sham treatment did not exhibit baseline retinal ischemia, but ASN developed during the follow-up period.
Twelve eyes had more than 10 disc areas of retinal capillary non perfusion on the baseline FA images. Of these, eight (66.67%) developed ASN (five eyes in the sham group [p=0.02]and three eyes in the triamcinolone group [p=0.14]). Four eyes displayed the angiographic criteria of ischemia, but ASN did not develop (three eyes randomized to IVB and one eye randomized to IVTA) Table 3.
Table 3 Primary outcome
| Sham (n=8) | Bevacizumab 1.25 mg (n=11) | Triamcinolone acetonide 1 mg (n=9) | Among-group p value* | |
|---|---|---|---|---|
| Clinical findings of retinal ischemia**(VA <20/200 + cotton-wool spots + afferent pupillary defect) | ||||
| n | 3 | 5 | 2 | |
| ASN, n (%) | 3 (100) | 0 | 2 (100) | |
| P | 0.17 | 0.056 | ||
| Fluorescein angiography**(>10 DA of capillary non-perfusion) | ||||
| n | 5 | 3 | 4 | |
| ASN, n (%) | 5 (100) | 0 | 3 (075) | |
| P | 0.02 | 0.140 | ||
| Total ASN, n (%) | 5 (50) | 0 | 3 (27.27) | 0.009 |
*P-values less than 0.05 were considered statistically significant;
**at the baseline visit. VA= visual acuity; DA= disk area; ASN= anterior segment neovascularization.
Functional outcomes at month 12
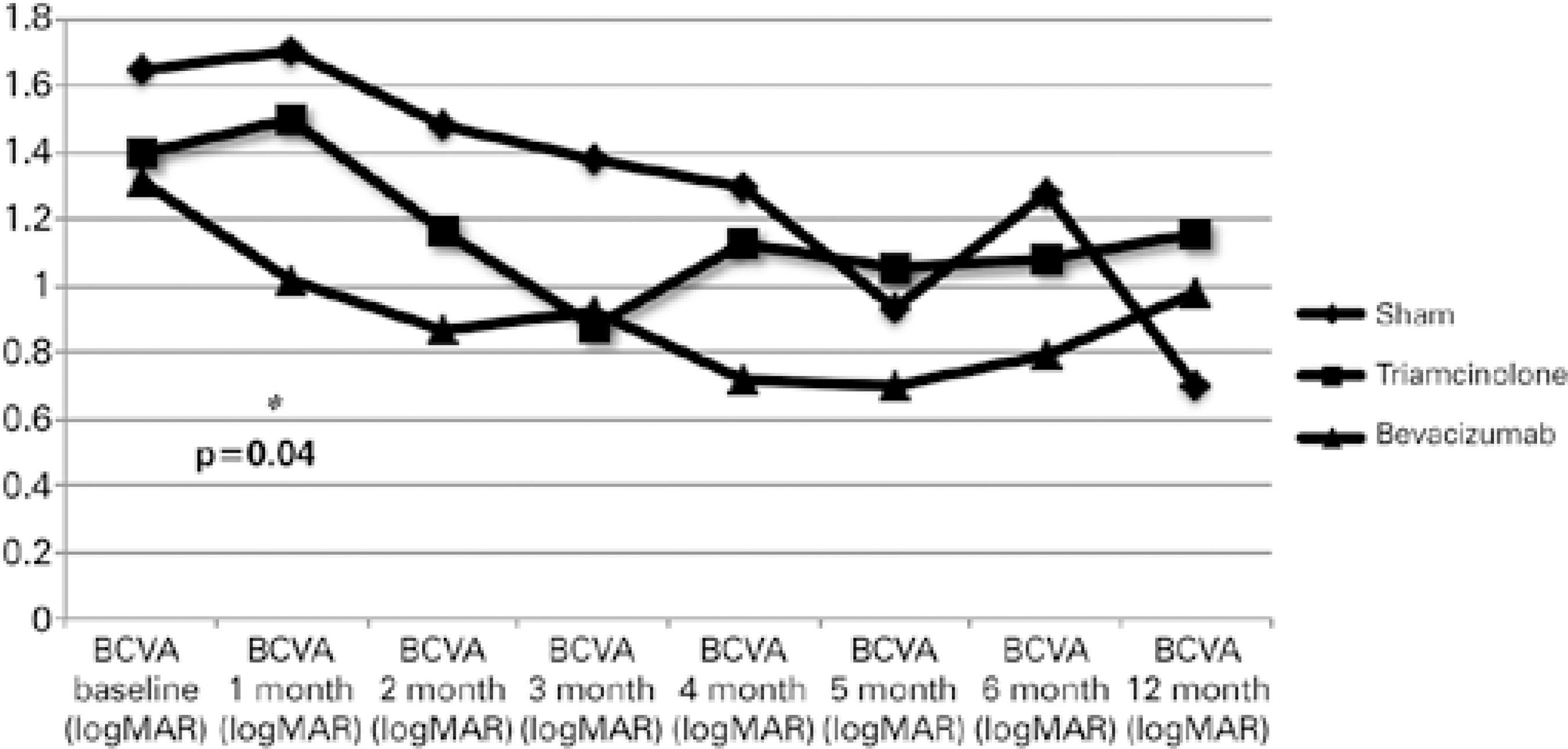
At months 6 and 12, the mean logMAR BCVA levels were 0.96 ± 0.67 (p=0.41) and 0.99 ± 0.53 (p=0.44), respectively. The mean change in BCVA during the first 12 months in the groups is shown in figure 2. BCVA differed significantly (p<0.05) among the groups only at month 1. Macular ischemia was observed in 13 eyes, including four (40%), five (45.45%), and four (28.57%) eyes randomized to sham, IVTA, and IVB treatment (p=0.84), respectively.
Anatomic outcomes at month 12
At months 6 and 12, the mean CFTs were 345.4 ± 182.87 (p=0.39) and 346.17 ± 195.92 (p=0.35), respectively. The mean changes in CFT on SD-OCT during the first 12 months in the groups are shown in figure 3. CFT did not differ significantly among the groups during this period.
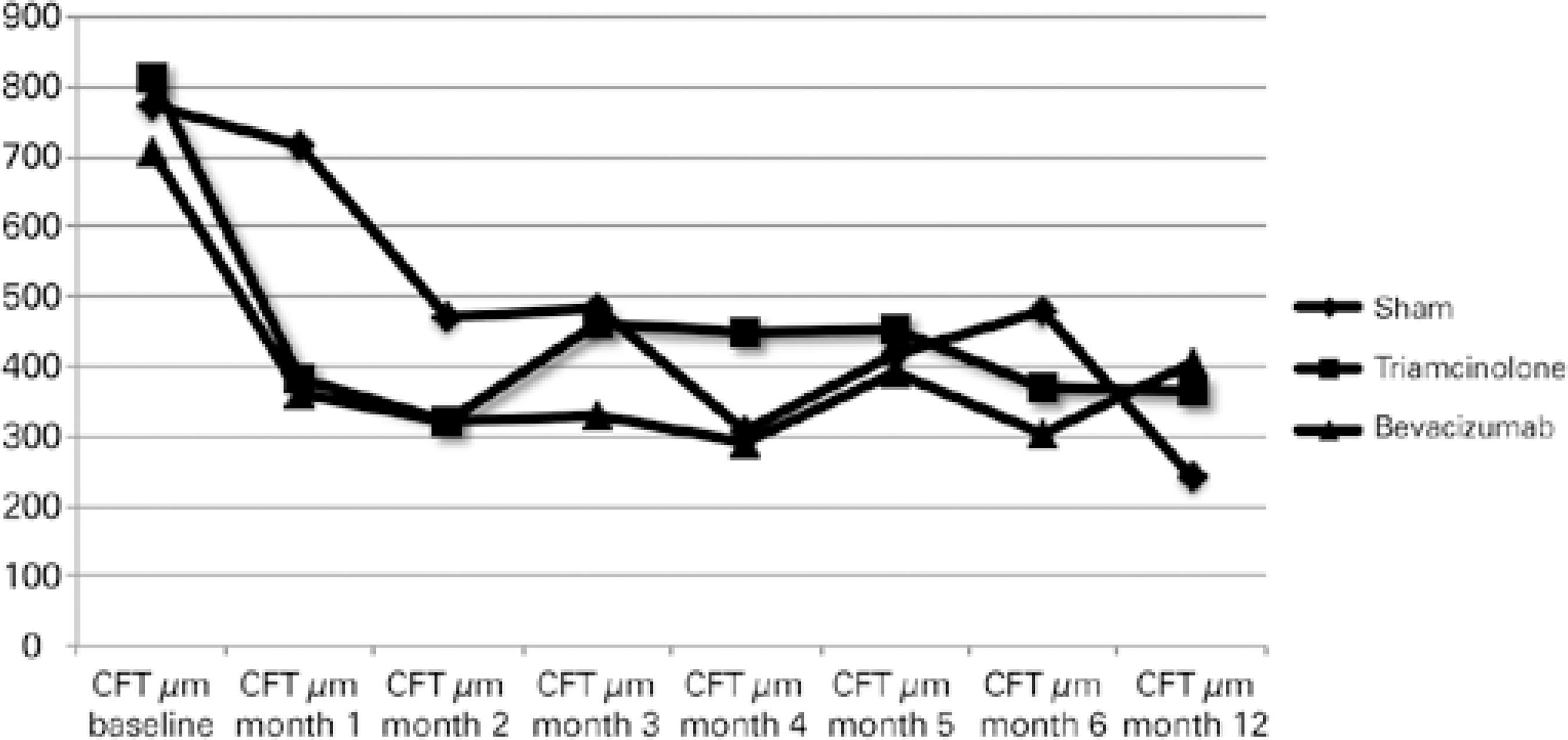
Figure 3 Mean change from the baseline central foveal thickness (CFT) over time to month 12. CFT did not differ significantly among the groups over the 12 months.
Safety outcomes at month 12
Seven (20%) eyes developed or exhibited worsening of cataracts (p=0.31). Ten patients required topical antiglaucomatous drops for increased IOP, none of whom had uncontrolled IOP. The differences among the groups did not reach significance (p=0.41). One patient discontinued follow-up 3 months after inferior paresis that required hospitalization. The patient was diagnosed with Miller Fisher syndrome. Another patient with cardiomyopathy related to Chagas disease required pacemaker implantation during follow-up.
All patients with ANS were referred for scatter PRP. NVG developed in one patient despite laser treatment, and surgery was needed to control IOP.
DISCUSSION
CRVO is an important cause of irreversible visual loss worldwide. The natural disease history has a poor prognosis that is proportional to the degree of retinal ischemia(5). Macular edema is an important cause of visual loss in these patients, and the benefits of intravitreal injections have been reported(11-14). Other possible complications related to CRVO are ASN and NVG. The cumulative incidence of NVG is 40% in ischemic CRVO and 10% in nonischemic CRVO(6). Despite the fact that many studies reported the benefits of intravitreal medications for improving BCVA and macular edema, there is little information about the impact of treatment on the natural history of ASN.
This study was a prospective analysis of different treatments for macular edema after CRVO with focus on preventing ASN and NVG.
Differentiating between ischemic and nonischemic CRVO may be challenging in the early stages. Clinical features such as initial BCVA worse than 20/200, APD, and cotton-wool spots are suggestive of ischemic CRVO and can predict prognosis(3,5,22-25). The presence of extensive nonperfused capillary areas in the FA images is a good indicator of retinal ischemia(5,6), but this can be difficult to assess while setting CRVO with substantial intraretinal hemorrhages. Although electroretinogram (ERG) is a good indicator of retinal ischemia in CRVO(23,26-28) and is predictive of iris neovascularization(29,30), it is expensive and not always available in daily clinical practice. Considering the difficulty of performing ERG, we correlated the clinical data that suggested ischemic CRVO with the development of ASN. Thus, it is possible to assess which patient groups may benefit from each macular edema treatment to prevent this complication.
In this study, ASN developed in eight (22.86%) patients. Ramezani et al. reported an incidence as high as 50% at 6 months after CRVO in a study in which the CRVO subtype was unclassified(30). In the CVOS, which considered eyes initially categorized as nonperfused or indeterminate, 35% of eyes developed ASN, compared with 10% of eyes initially categorized as perfused(5).
Five (62.5%) eyes that developed ASN were randomized to sham treatment, and three (37.5%) eyes were randomized to the IVTA group. No eyes in the IVB group developed ASN during the first 6 months of follow-up (p=0.009).
In this study, ASN developed after an average of 59.75 ± 42.79 (65.6 ± 54.22 days in the sham group; and 50 ± 17.32 days in the triam cinolone group).
Twelve patients had baseline FA images classified as ischemic. Among these patients, five, four, and three eyes were randomized to sham, IVTA, and IVB treatment, the last of which was the only group with an ischemic angiographic pattern that did lead to the deve lopment of ASN.
The CRUISE study reported iris neovascularization in only 12 of 390 eyes and NVG in two eyes. Only three patients treated with ranibizumab in that study developed iris neovascularization, and none developed NVG; however, the study excluded patients with APD and included patients with BCVAs ranging from 20/40 to 20/320. In this study, BCVA and APD were not the exclusion criteria, and our sample probably included patients with more severe retinal ischemia compared to the CRUISE study.
The functional and anatomic outcomes in this study were worse than the SCORE and CRUISE results. BCVA in patients treated with anti-VEGF injections was significantly (p<0.05) better than that in the other groups only at month 1. CFT measured on SD-OCT images in these patients was not significantly better than that in the other groups. These results are probably related to the small sample size and the inclusion of patients with a worse prognosis compared to patients in other trials.
At the end of the 12-month follow-up period, we found similar ocular adverse events (cataract and ocular hypertension) rates compared with previous studies(11). Endophthalmitis did not develop during the study. No SAEs reported were related with the use of the study medications.
Although this was a prospective, randomized, sham-controlled study, our study had limitations, including the small sample size in each group, which might have compromised the statistical power to detect differences.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

