INTRODUCTION
Keratoconus (KC) is a bilateral, non-inflammatory, and usually asymmetrical disease of the eye. The prevalence in the general population is between 4/1000 and 6/1000(1). Characteristics of KC include decrea sed biomechanical strength of the cornea and stromal thinning, which gradually decreases corneal thickness and induces irregular astigmatism, myopia, corneal scaring, and visual acuity(2,3). Corneal collagen crosslinking (CXL) has been considered for more than a de cade as the only method available to increase corneal biomechanical power and treat KC(4). In CXL, riboflavin, activated by ultraviolet A (UVA) light, increases corneal collagen fiber connections, which stabilize biomechanical measures(5,6).
One year after CXL apex reduction has been previously reported; however, there is no short-term stabilization analysis. In addition, there are few studies that have compared the effects of CXL on gender(7). The aim of our study was to determine the stability and effects of CXL at 3 and 12-month follow-ups, as well as to compare KC progression between genders, as performed according to the classic Dresden protocol(6).
METHODS
This was a non-randomized, retrospective observational study approved by the Research Ethics Committee of the Institute of Medical Research of the Evangelical School of Paraná, and this study adhered to the tenets of the Declaration of Helsinki.
The study comprised a total of 100 eyes from 75 patients diagnosed with progressive KC and underwent CXL between January 2011 and July 2014 at the Eye Hospital of Paraná, Brazil. Inclusion criteria were; clinically evident KC, grades 1-3 according to Amsler-Kru meich's classification(8), documented KC progression, and spectacle-corrected distance visual acuity (SCDVA) ≥0.48 logMAR (logarithm of the minimum angle of resolution). Of the patients with KC grade 3, only thickness values ≥400 µm at the thinnest point were included. The diagnosis of KC progression was defined as an increase in the maximum keratometry (Kmax) value by at least 0.75 diopter (D)(9) and/or an increase in the manifest cylinder greater than 0.75 D for at least two consecutive measurements in the past 6 months.
Exclusion criteria were; KC grade 4 according to Amsler-Kru meich's classification(8), any previous eye surgery, any ocular co-morbidity, history of herpetic eye disease, an ocular or systemic condition that could interfere in the stability of the lacrimal film (i.e., moderate and severe dry eye, eyelid abnormalities, chalazion, meibomitis, ble pharitis, and corneal scars), or use of contact lenses within the 4 weeks before the first appointment.
All patients in the study underwent a detailed ophthalmolo gical examination that included: SCDVA measurement with Snellen charts, slit-lamp examination, applanation tonometry, and dilated fundoscopic examination. Visual acuity in Snellen format was conver ted to the logMAR. Corneal topography was performed using a computerized corneal topographer with Placido disk Tomey - TMS-4 model (Tomey Corp., Nagoya, Japan). All eyes in this study had a preoperative topography within 1 month before CXL treatment. The corneal topography parameters analyzed were: flattest keratometry reading (K1), steepest keratometry reading (K2), average keratometry (Km), topographic astigmatism (dK), and Kmax.
CXL was performed using 0.1% riboflavin (in 20% Dextran T 500) and UVA (wavelength=370 nm and irradiance=3 mW/cm2) for 30 min under sterile conditions. The UV-X 1000 machine (IROC Innocross AG, Zurich, Switzerland) and the Innocross-R riboflavin isotonic solution (0.1% riboflavin 5-phosphate plus 20% dexDtran T 500 in 2 mL syringes) were used. Three experienced surgeons performed the procedure. The topical anesthetic 0.5% proxymetacaine hydrochloride (Alcaine; Alcon Laboratories, Inc.) was used for all patients. Epithelial tissue was removed in an 8.0 mm diameter area to allow penetration of riboflavin into the corneal stroma. Thereafter, 0.1% riboflavin was applied (1-2 drops every 5 min) to the cornea for 30 min before irradiation to allow sufficient saturation of the stroma.
Corneal soaking with riboflavin was assessed, and then the cornea (8 mm in size) was exposed to UVA light for 30 min as previously stated. Throughout UVA exposure, riboflavin solution was applied (1-2 drops every 5 min). Upon completion of treatment, the eye was washed with balanced salt solution, and antibiotic eye drops (0.5% moxifloxacin) and steroid eye drops (0.1% dexamethasone) were applied. A bandage contact lens was placed in the eye until complete re-epithelialization. Follow-up examinations were performed at 1 week and 1, 3, and 12 months after CXL. The SCDVA and topographic values were recorded at 3 and 12 months and analyzed with preoperative data.
Statistical analysis was performed using the Statistical Package for Social Sciences software version 16.0 (SPSS Inc., Chicago, USA). Each variable was compared between different follow-up times using the Wilcoxon test. The Mann-Whitney U test compared variables between genders. Visual acuity results were converted into logMAR values before averaging and performing statistical calculations. Data were recorded as mean ± standard deviation. P<0.05 was considered statistically significant.
RESULTS
A total of 100 eyes from 75 patients that underwent CXL were in cluded in this study. There were 50 (66.6%) males and 25 (33.4%) females. The mean age was 19.9 ± 5.61 years, ranging from 12 to 30 years. All patients were followed up at 3 and 12 months, with a 0% dropout rate. The mean preoperative SCDVA was 0.18 ± 0.19 logMAR, and 1-year post-CXL SCDVA was 0.13 ± 0.16 logMAR (p<0.05), a statistically significant change. The mean preoperative corneal thickness was 492.18 ± 39.91 µm and 1 year after CXL was 481.54 ± 42.17 µm. This difference was not significant (p>0.05).
Table 1 shows the mean topographic preoperative and 3 and 12-month postoperative outcomes for all patients. One year after CXL, there was a significant regression of K1, K2, Km, and Kmax when compared to the preoperative values. There was a significant reduction in average K2 and Kmax values (p<0.05) 3 months after CXL. The Kmax value had a reduction of 0.68 D after 3 months and 0.87 D after 12 months when compared to preoperative data. Regarding the dK parameter, there was no statistically significant difference at 3 months and 12 months following CXL.
Table 1 Preoperative, 3 and 12-month postoperative topographic and visual outcomes in 75 patients (100 eyes) who underwent corneal collagen crosslinking (CXL)
| Parameters | Preoperative | After 3 months | After 12 months |
|---|---|---|---|
| K1 | 44.65 ± 2.40 D | 44.61 ± 2.37 D | 44.46 ± 2.38 D |
| (p=0.5) | (p=0.02) | ||
| K2 | 48.84 ± 4.07 D | 48.64 ± 3.83 D | 48.54 ± 3.93 D |
| (p=0.03) | (p=0.02) | ||
| Km | 46.72 ± 2.97 D | 46.63 ± 2.89 D | 46.50 ± 2.95 D |
| (p=0.18) | (p=0.03) | ||
| dK | 04.20 ± 2.85 D | 04.06 ± 2.67 D | 04.10 ± 2.68 D |
| (p=0.07) | (p=0.24) | ||
| Kmax | 53.32 ± 5.92 D | 52.64 ± 5.29 D | 52.45 ± 5.65 D |
| (p=0.00) | (p=0.00) | ||
| SCDVA | 0.18 ± 0.19 logMAR | - | 0.13 ± 0.16 logMAR |
| (p=0.00) |
Variables= flattest keratometry reading (K1); steepest keratometry reading (K2); average keratoetry (Km); topographic astigmatism (dK); maximum keratometry (Kmax); and spectacle-corrected distance visual acuity (SCDVA). D= diopters and logMAR= logarithm of the minimum angle of resolution. Data are expressed as mean ± standard deviation.
There was no statistically significant relationship between patient gender and age. Upon comparing genders following CXL, as shown in table 2, there were no statistically significant differences related to the changes in Kmax, K2, and SCDVA. No complications were observed during the study period. No patient was lost to follow-up.
Table 2 Preoperative, 3, and 12-month post-corneal collagen crosslinking (CXL) topographic and visual outcomes according to gender
| Parameters analyzed | Gender | Preoperative | After 3 months | After 12 months |
|---|---|---|---|---|
| Kmax (D) | Male | 53.97 ± 5.38 (44.58 - 65.00) | 53.46 ± 4.93 (43.91 - 63.41) | 53.15 ± 5.14 (44.81 - 66.00) |
| Female | 52.13 ± 6.31 (43.54 - 64.89) | 51.21 ± 5.38 (43.76 - 64.88) | 51.27 ± 5.99 (43.02 - 64.63) | |
| (p=0.149) | (p=0.070) | (p=0.121) | ||
| K2 (D) | Male | 49.46 ± 4.03 (42.57 - 60.27) | 49.20 ± 3.87 (42.91 - 58.90) | 49.17 ± 3.99 (42.64 - 61.25) |
| Female | 47.56 ± 3.05 (42.61 - 56.69) | 47.34 ± 2.47 (42.89 - 51.87) | 47.19 ± 2.47 (42.52 - 51.50) | |
| (p=0.044) | (p=0.051) | (p=0.045) | ||
| SCDVA | Male | 0.20 ± 0.20 (0.00 - 1.00) | 0.16 ± 0.17 (0.00 - 0.70) | |
| (logMAR) | Female | 0.11 ± 0.13 (0.00 - 0.48) | 0.08 ± 0.13 (0.00 - 0.48) | |
| (p=0.068) | (p=0.030) |
Variables= maximum keratometry (Kmax); steepest keratometry reading (K2); and spectacle corrected distance visual acuity (SCDVA). D= diopters and logMAR= logarithm of the minimum angle of resolution. Data are expressed as mean ± standard deviation (minimum - maximum).
Three months after CXL, of the 100 eyes examined and compared preoperatively, 86 (86%) eyes remained stable or had decreased corneal apex values, and 14 (14%) eyes experienced Kmax progression. Of these 14 eyes, seven (50%) kept progressing and seven (50%) returned to basal or improved Kmax values at 12 months following CXL.
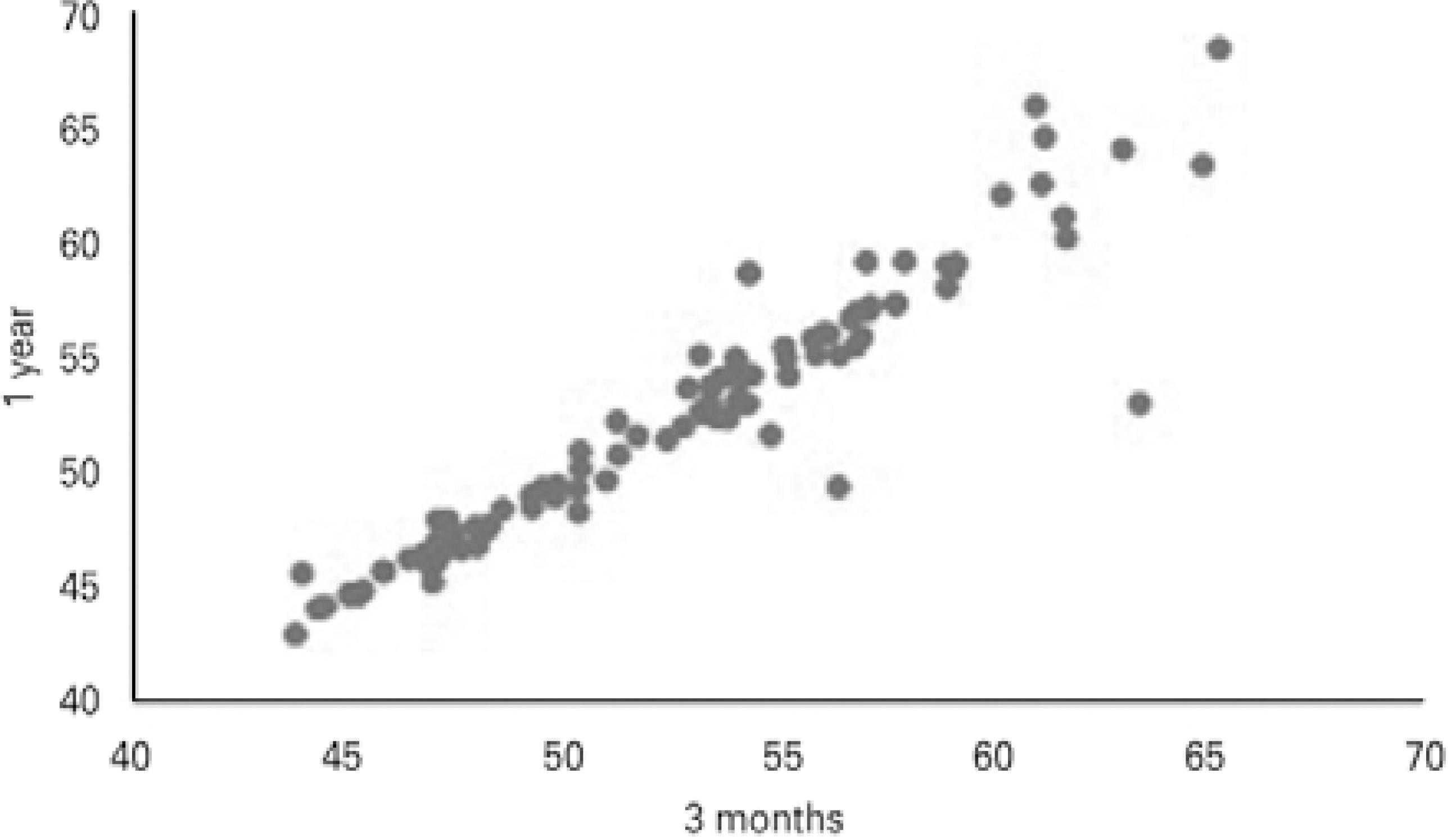
Twelve months after CXL, of the 100 eyes examined, 82 (82%) eyes had stabilized or had decreased apex values, and 18 (18%) eyes had progression of the corneal apex (mean 1.37 ± 1.08 D) compared with preoperative values. Of the 18 eyes, seven eyes (38.9%) already displayed progression at 3 months following CXL (mean 1.54 ± 0.73 D), and 11 eyes (61.1%) were stable at 3 months and progressed afterwards. Figures 1 and 2 show Kmax variation when data from the preoperative period were compared with the data 3 and 12 months after CXL, respectively. Figure 3 shows the comparison of Kmax variation between 3 and 12 months after CXL.
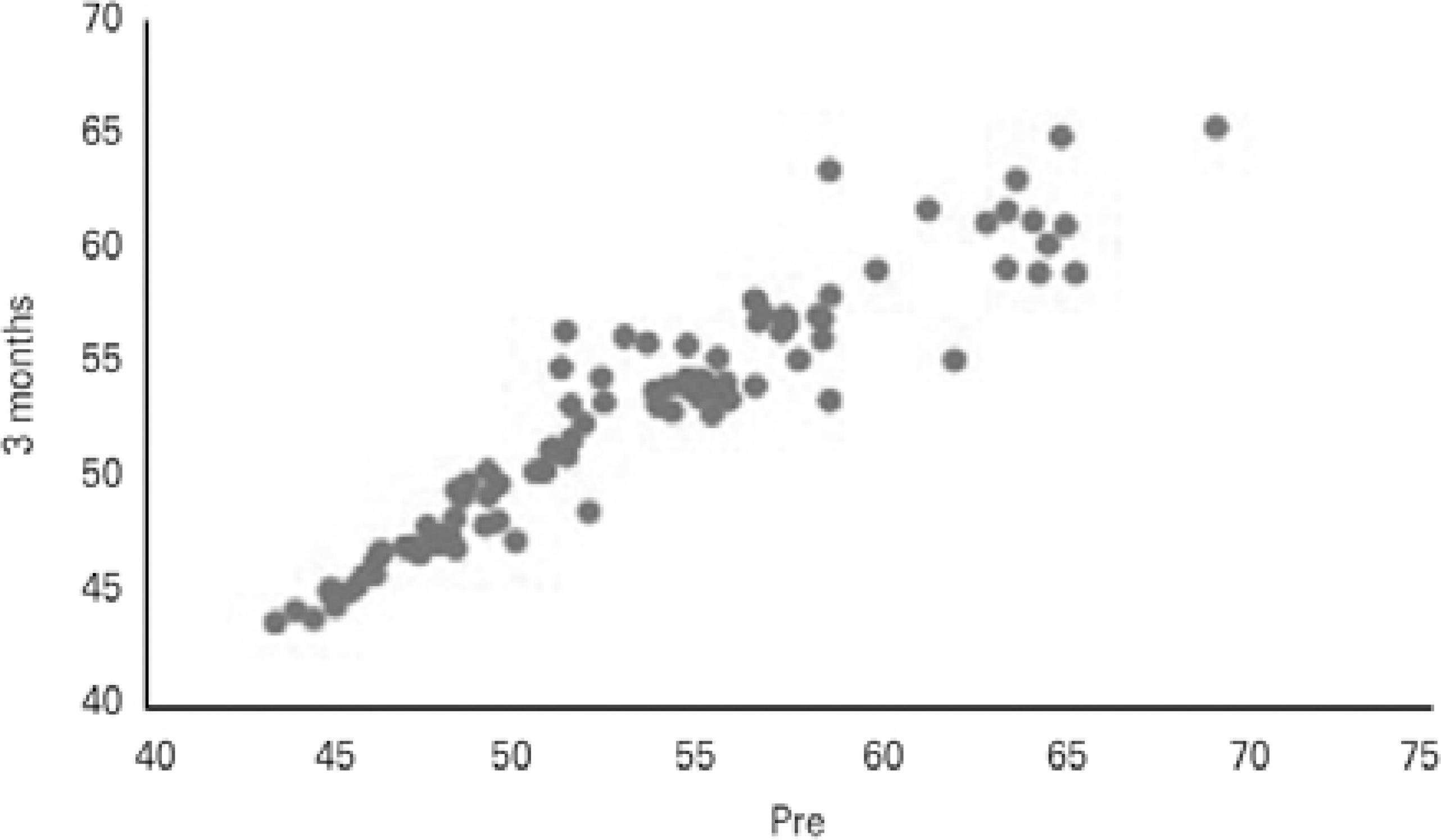
Figure 1 Comparisons between preoperative and 3-month follow-up maximum keratometry (Kmax) values in diopters (D).
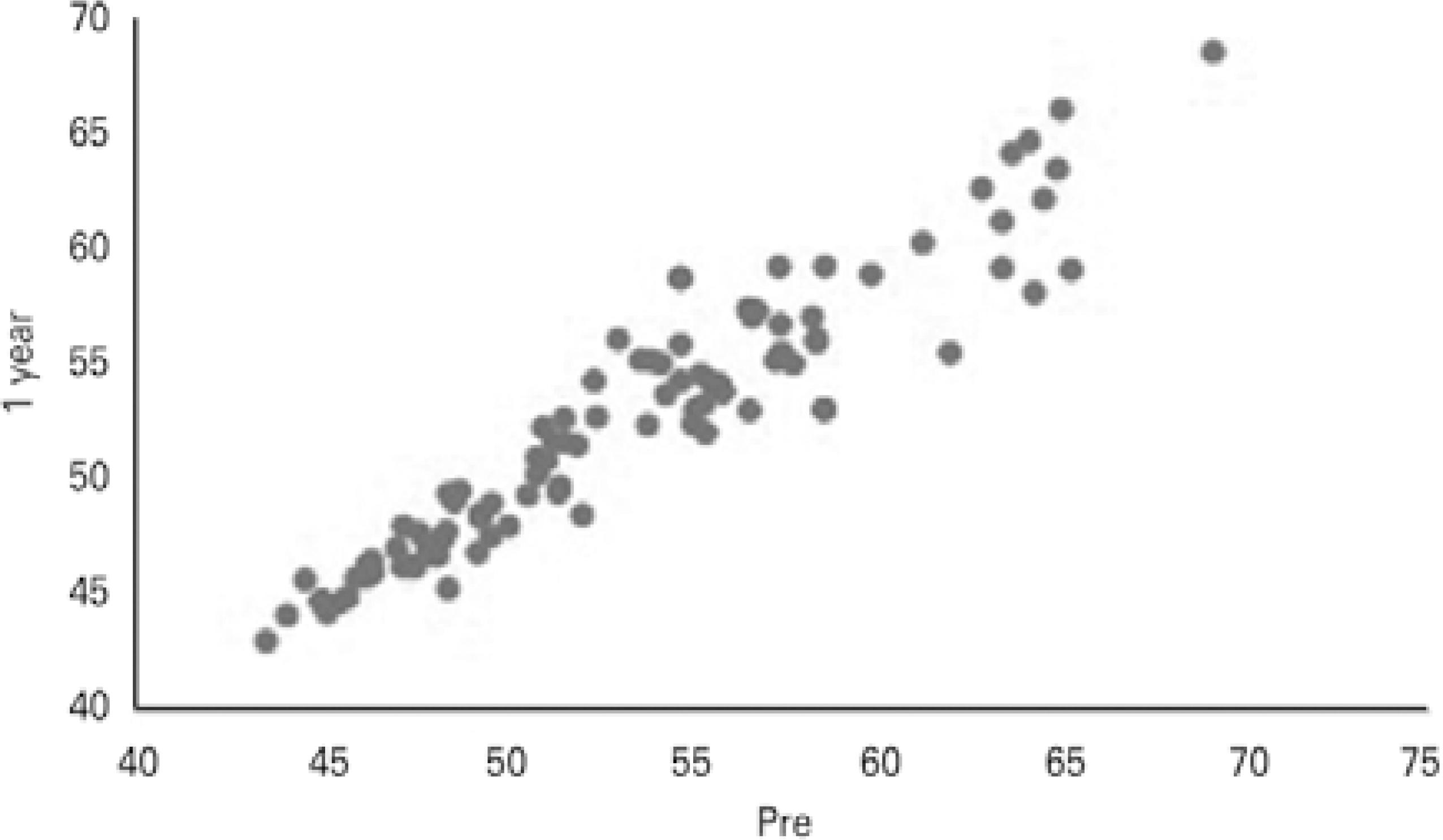
Figure 2 Comparisons between preoperative and 12-month follow-up maximum keratometry (Kmax) values in diopters (D).
DISCUSSION
Over the last decade, there have been proposed new and improved interventions for KC intended to considerably decrease transplantation rates and arrest disease progression(10). CXL is, at present, the only treatment that can slow down or even stop KC progression. The purpose of CXL is to stabilize the underlying disease process, and corneal topography (Kmax and Km) is considered one of the key outcome measures(7).
Spoerl et al. first tested the effects of a combination of riboflavin, used as a photosensitizer, and UV light in laboratory experiments. Increase in corneal rigidity was reported in-vitro in both porcine and human corneas(11,12). This was followed by the first pilot clinical trial carried out by Wollensak et al. in Dresden. At follow-ups ranging between 3 and 47 months, progression of KC stopped in all 23 treated eyes. An average reduction of 2.01 D in Kmax, 1.14 D regression in refractive error in 16 eyes, and visual acuity improvement in 15 of 22 eyes were observed(6). In our study of 100 eyes, we found a mean reduction of 0.87 D in Kmax at the 12-month follow-up, and this value was the most statistically significant decrease of all the analyzed pa rameters. The SCDVA, in our results, improved in 33% of treated patients and remained stable in 62% of patients.
Numerous studies have evaluated the clinical effect of CXL in the treatment of KC. Wittig-Silva et al.(13) performed a randomized, controlled clinical trial using CXL in 66 eyes of 49 KC patients. Their results showed that Kmax was significantly decreased at all follow-up time points, with an average decrease of 1.45 D at the 12-month follow-up. A trend toward improvement in SCDVA was also observed. Analysis of the control group that did not undergo CXL showed a continuous deterioration in Kmax and SCDVA. In our present study, we demonstrated a statistically significant stability of KC 3 months after CXL, particularly for the K2 and Kmax parameters, and after 12 months regarding the K1, K2, Km and Kmax values. There was no statistically significant difference 3 months after CXL for the K1 and Km parameters.
Other studies showed a significant improvement in SCDVA and keratometric parameters. For example, Lamy et al.(14) investigated 68 KC eyes in a 2-year follow-up study and showed an improvement of 0.16 in SCDVA and 0.61 D in Kmax. In 2013, Viswanathan and Males(15) analyzed 51 KC eyes and demonstrated Kmax improved by 0.96 ± 2.33 D (p=0.005), and a logMAR SCDVA improvement of 0.05 ± 0.13 (p=0.04). In 2011, Hersh et al.(16) studied 49 KC eyes and noted that SCDVA improved from 0.35 ± 0.24 to 0.23 ± 0.21 (p<0.001) and Kmax improved by 2.0 ± 4.4 D (p=0.002).
In our study, at 1-year post-CXL, 18 eyes (18%) had Kmax progression, and 82 eyes (82%) had stabilized or showed improved apex values. Sloot et al.(17) demonstrated that the overall percentage of failure after CXL was 9%, which was similar to previous findings(16,18) that estimated a failure rate of 8%-10%. In these studies, failure was defined as an increase of ≥1.0 D in Kmax. This was different from our study, in which we considered progression to be Kmax value ≥0.75 D.
Arora et al.(19), in a prospective and non-randomized comparative clinical intervention study, analyzed 30 KC eyes after CXL treatment and concluded that CXL was more effective in improving the refrac tive and topographical parameters at 1 year when performed early in the course of the disease. However, in our study, we did not analyze the time from diagnosis to treatment. Given our study design, we found that age was not statistically significant in determining CXL outcomes. In addition, we did not find a positive relationship between the efficiency of CXL treatment and gender, corroborating the findings of previous studies. Toprak et al.(20) in their retrospective study compared the postoperative changes in Kmax between gender subgroups and also reported no significant differences between males and females (p=0.501, p=0.183, respectively).
In conclusion, CXL promoted the stability of KC by maintaining keratometric values and SCDVA after a 12-month follow-up. Our re sults reinforced previous studies highlighting CXL efficiency in KC patients. The stability of the apex of KC after CXL may be achieved as early as 3 months after the procedure. We found no statistically significant differences in keratometric and refractive values between female and male patients after CXL. Future studies consisting of a larger number of patients and longer follow-up periods are needed to confirm the findings presented herein.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

