INTRODUCTION
Retinoblastoma (RB) is the most common intraocular tumor in childhood(1,2), and the vast majority of cases (about 80%) are diagnosed before the age of three(3). Retinoblastoma originates from a neurosensitive retina, and despite extensive study for many decades, its origin remains controversial(4,5). Various studies posit that the retinoblastoma cell could be a primitive multipotent cell, a photoreceptor/glial dual differentiation cell, or strictly a neuronal cell(5-10). Others propose the theory that the original cell may be a differentiated photoreceptor, specifically a cone(6,8,11,12).
Several molecules have been identified that are expressed only at specific times during retinal development, suggesting utility as markers for cell type and degree of differentiation(13-17). SOX-2 is a 317 amino acid protein belonging to the family of strong sex determining region Y-box (SOX) transcription factors crucial for maintaining multipotency of neuroglial stem cells. SOX-2 is strongly expressed by neuroepithelial cells during early development of the central nervous system(18). Microtubule-associated protein 2 (MAP2) is a 280 kD protein highly concentrated in the soma and dendrites of mature neurons(13,14,19). Glial fibrillary acidic protein (GFAP) is a marker of mature glia, including retinal glia. Studies of expression in retinoblastoma(7-10) have detected GFAP in reactive Mϋller cells (reactive astrocytes) surrounding the tumor(13).
In the current study, a series of enucleated and archived retinoblastoma samples and two RB cell lines (WERI-1 and Y79) were immunostained for markers of immature neural cells (SOX-2), mature neuronal cells (MAP2), and astroglial cells (GFAP) to examine cell origin and the relationship between marker phenotype and histopathological prognostic factors.
METHODS
Thirty-nine tissue samples of retinoblastoma added to the Henry C. Witelson Ocular Pathology Laboratory and Registry files at McGill University (Montreal, Canada) from the Instituto Anatomopatológico "Dr. José A. O'Daly" of the Universidad Central of Venezuela were selected, and clinical/epidemiological data (age, sex, and eye side) collected. No long-term clinical data were available because the request forms did not contain the information or links to access patient medical records. Thus, no comparisons that allowed determination of prognosis and survival were conducted.
Some morphological characteristics, such as differentiation status of the tumor (differentiated or un-differentiated), choroidal/scleral and optic nerve level of invasion (according to the Khelfaoui criteria) (Table 1)(15), presence of vitreous seeding, vascular basophilia, and anterior chamber invasion were also recorded and re-evaluated.
Table 1 Choroidal/scleral and optic nerve involvement according to the criteria of Khelfaoui et al.(15)
| Choroidal/scleral involvement | Definition |
|---|---|
| No choroidal involvement | |
| Minimal involvement | Tumor cells having destroyed Bruch’s membrane without invading the choroid, with a maximum of three microscopic cell clusters |
| Massive involvement | Any choroidal involvement that is not minimal |
| Intrascleral involvement | Any scleral involvement |
| Extrascleral involvement | (i.e., microscopic orbital involvement) |
| Optic nerve (ON) involvement | |
| No optic nerve involvement | |
| Prelaminar involvement | Anterior to the lamina cribrosa |
| Postlaminar without invasion of the ON resection line or subarachnoid space |
Within or beyond the lamina cribrosa |
| Invasion of the resection line and/or subarachnoid space |
|
Two human RB cell lines (WERI-1 and Y79, American Type Culture Collection, Manassas, VA, USA) were also examined for marker phenotype. These cell lines were cultivated until use according to standard procedures.
Immunohistochemistry and immunocytochemistry were conducted using an automated Benchmark® slide staining system (Ventana Medical System, Inc.; Tucson, Arizona). Immunostaining modules for SOX-2, MAP2, and GFAP were used according to protocols and instructions provided by the supplier (Ventana Medical System, Inc.). All immunostaining protocols were based on a streptavidin-biotin complex(20-23).
For positive controls, SOX2 staining was confirmed in fetal retina cuts, MAP-2 staining in normal retina of the studied eyeballs, and GFAP in normal retina and optic nerve of the studied eyeballs. For negative controls, the primary antibody was omitted.
The staining intensity for all antibodies was graded from 0-3, where 0= negative staining, 1+= weak staining, 2+= moderate staining, and 3+= strong staining. Positive staining was defined as 2+ or 3+ intensity in the nucleus and cytoplasm for SOX-2 and in the cytoplasm for MAP2 and GFAP, while staining intensity of 0+ or 1+ was considered negative.
Specimens were independently analyzed by two experienced ocular pathologists (M.E.O. and M.N.B. Jr.) who rated the samples on two different occasions. They were not aware of prior medical history, the results of previous assessments, or evaluation by the other observer. Conflicts in assessment were resolved by mutual agreement between the observers, and the final decision was used for statistical analysis.
For statistical analysis, frequency and percentage of nominal and ordinal variables were calculated. Nominal variables were compared by Pearson Chi-square test. Results were considered significant when the p value was less than 0.05. The relationships between intensity of immunostaining and various histopathological parameters were assessed by correlation analysis. A p value less than 0.05 was considered a statistically significant correlation.
The study protocol was approved by the ethics committees of the Federal University of São Paulo (Annex) and McGill University (McGill University Health Centre, Montreal, Canada). The use of material from the Instituto Anatomopatológico (Caracas, Venezuela) was authorized by the originating section.
RESULTS
The thirty-nine archived tissue samples were obtained from patients between 4 and 108 months of age (mean ± SD, 24 ± 21.8 months; 48% female), with most cases in the range of 4 to 12 months. The series included 18 cases of well/moderately differentiated retinoblastoma (46.2%) and 21 cases of poorly differentiated retinoblastoma (53.8%). The level of choroidal/scleral invasion was stage I in 12 cases (30.7%), stage II in 7 (18%), stage III in 13 (33.3%), stage IV in 5 (12.8%), and stage V in 2 cases (5.2%). The degree of optic nerve invasion was stage I in 9 cases (23%), stage II in 15 (38.5%), stage III in 11 (28.2%), and stage IV in 4 cases (10.3%). Vitreous seeding was observed in 28 cases (71.8%) and invasion of retinoblastoma tumor cells into the anterior chamber was observed in 8 cases (20.5%). Basophilia of vascular walls, inner limiting membrane of the retina, and fibers of Zinn's zonule was observed in 18% of cases.
Thirty-eight cases showed positive nuclear and cytoplasmic immunostaining for SOX-2 (97.4%) and 23 cases (59%) for MAP2. Both SOX-2 immunostaining (Figure 1) and MAP immunostaining (Figure 2) were also observed in well-differentiated tumor cells corresponding to foci of retinocytoma. Most retinoblastoma cases also showed intense cytoplasmic immunostaining for GFAP in isolated astrocytes within the tumor and in Mϋller cells of uncompromised retina, but tumor cells were completely negative for this marker in all cases (Figure 3).

Figure 1 Immunoexpression of SOX-2 in retinoblastoma. A) Positive staining in tumor cells forming a pseudovascular rosette (arrow) (×400). B) Intense positivity in tumor cell cytoplasm from a differentiated retinoblastoma with fleurettes (arrowheads) and Flexner-Wintersteiner rosettes (arrows) (×200). C) Positive immunostaining in an undifferentiated retinoblastoma. Adjacent endothelial cells (e) are negative (×200). D) Intense staining in an undifferentiated retinoblastoma located close to non-tumoral retinal internal limiting membrane and the nerve fiber layer (×320).

Figure 2 Immunoexpression of MAP2. A) Positive staining in tumor cells forming a pseudovascular rosette (arrow) (×320). B) Differentiated retinoblastoma with fleurettes (arrowheads) and Flexner-Wintersteiner rosettes (arrow) with intense MAP2 positivity in tumor cell cytoplasm (×400). C) Moderate positivity in an undifferentiated retinoblastoma (×400). D) Negative staining in an undifferentiated retinoblastoma (×320).
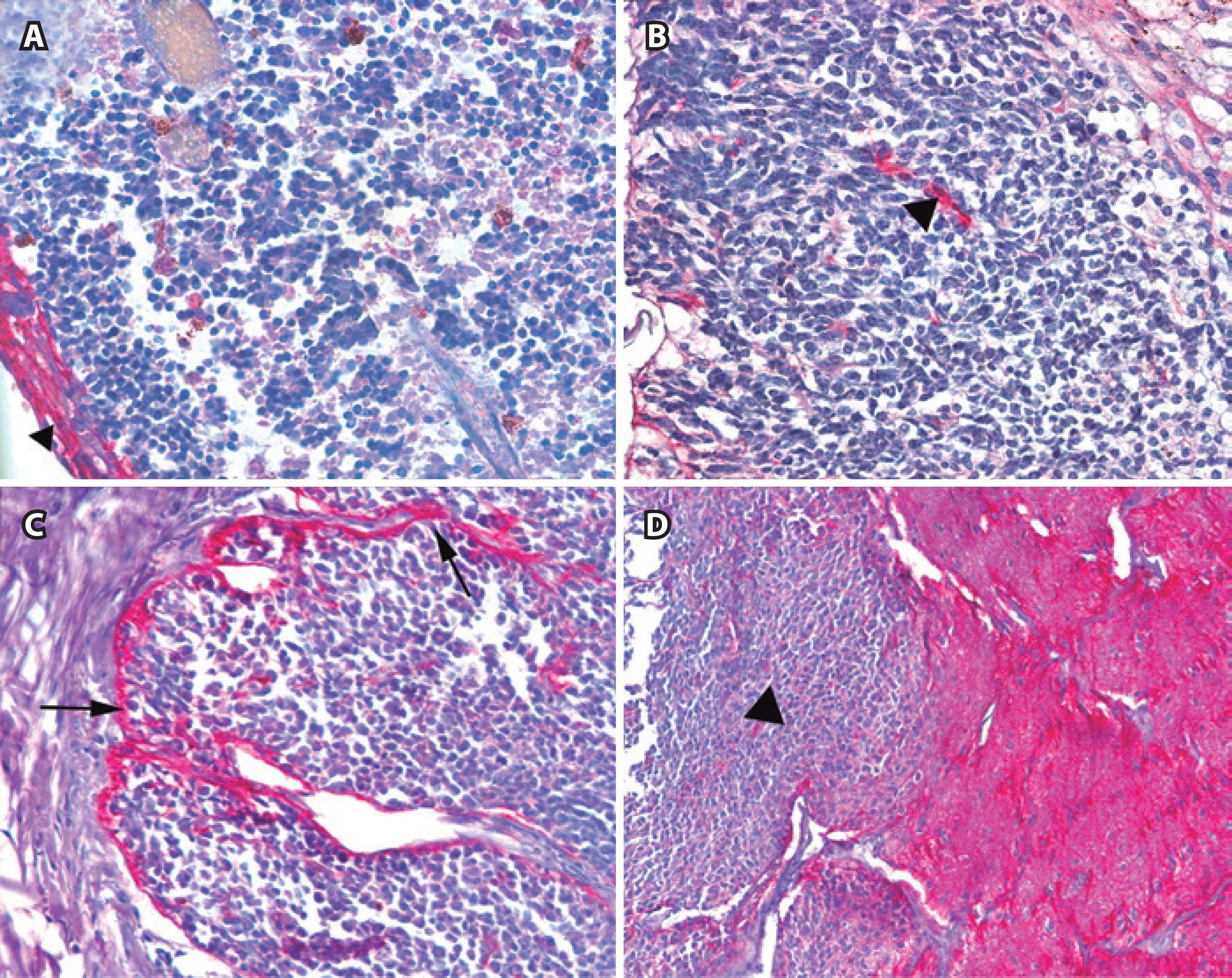
Figure 3 Immunoexpression of GFAP. A) Negative immunostaining in tumor cells of a differentiated retinoblastoma but intense positivity in peritumoral glial cells (arrowhead) (×200). B) Negative immunostaining in tumor cells of an undifferentiated retinoblastoma with intense positivity in peritumoral glial cells (arrowhead) (×320). C) Negative immunostaining in tumor cells of an undifferentiated retinoblastoma with intense positivity in both peritumoral and sporadic intratumoral glial cells (arrows) (×200). D) Tumor cells located at the optic nerve (left side-arrowhead) are negative for GFAP in an undifferentiated retinoblastoma. Nerve fibers are GFAP positive (right side) (×100).
In addition to retinoblastoma tissue, SOX-2 and MAP2 immunoreactivity were observed in normal retinal pigment epithelium, inner segments of photoreceptors, outer and inner plexiform layers of the retina, ganglion cells, nerve fiber layer, optic and ciliary nerves, non-pigmented epithelium of the ciliary body, and corneal and limbic epithelia. The optic nerve and ciliary nerves also showed positive immunostaining for GFAP. These extratumoral staining results confirm that all three antibodies efficiently recognize the intended antigens.
There were statistically significant positive associations between patient age and both SOX-2 immunostaining (p=0.0045) and MAP2 immunostaining (p=0.0311). In contrast, no other demographic or clinical variable was significantly associated with SOX-2 or MAP-2 expression status.
SOX-2 was observed in cytoplasm and nucleus of both WERI-1 and Y79 retinoblastoma cell lines, with more intense immunostaining in the WERI-1 line. MAP2 staining was equivalently strong in the cytoplasm of WERI-1 and Y79 cell lines, while GFAP immunostaining was negative in both cell lines (Table 2).
DISCUSSION
Identifying the origin of retinoblastoma will advance our understanding of how cellular context influences the probability of cancer initiation and progression by specific mutations (e.g., chromosome 1q and 6p gain and 16q loss often associated with this tumor). Further, knowledge of cellular origin may guide treatment decisions and provide clues for improving existing therapies or developing new treatments. We found that almost all retinoblastoma samples were immunopositive for the immature neural cell marker SOX-2 and the majority of samples were immunopositive for the mature neuronal marker MAP2, including well-differentiated tumor cells. In contrast, none expressed the astroglial marker GFAP, suggesting that neuroblastoma originates from cells committed to the neuronal lineage.
During the last ten years, significant advances have been made in the epidemiology and clinical management of retinoblastoma, such as identification of histopathological markers and tumor suppressors like Rb protein and its associated pathways. However, the cellular origin of retinoblastoma is still controversial(4,13,24). Molnar et al.(9) studied seven retinoblastoma cases and observed that tumor cells stained positively for myelin-associated glycoprotein (GPAM), which is only expressed in Müller cells in normal retina, whereas the same cells were negative for neuron-specific enolase (NSE) and GFAP, suggesting Müller rather than neuronal or astroglial origin. An important factor to consider, however, is the possible existence of a mixed population of cells within a tumor. Tarlton et al. studied six retinoblastomas with a panel of 18 monoclonal antibodies covering a wide range of tissue types and found marked heterogeneity within each tumor, possibly due to multiple intratumoral foci of differentiation(25-27).
Many molecules are expressed only at specific times during retinal differentiation and can be used as specific markers for cell type and degree of differentiation. These include molecules expressed at high levels in immature retinal stem cells compared to mature retinal cells(13,28). In this study, we observed that retinoblastoma tumor cells were positive for SOX-2 and MAP2, with a higher percentage immunopositive for the marker of immature neural cells (SOX) than for mature neurons (MAP2), although the difference was not statistically significant. These results are consistent with phenotypic heterogeneity and indicate that retinoblastoma is composed of cells that express markers of both differentiated and undifferentiated cells(29). This heterogeneity should be considered when assessing the response of different cell types to potential therapeutic drugs.
Sakata et al. used nestin (a marker that decreases in expression with differentiation), HES-1 (a transcription factor regulating neuronal development), and Chx10 (a regulator of proliferation and bipolar cell development) to mark immature stem cells, and photoreceptor-specific nuclear receptor (PNR), GFAP, and MAP2 as markers of mature retinal cells. Five cases studied were positive for MAP2, while none were positive for GFAP, suggesting that these retinoblastomas originated from a post-mitotic neuronal cell lineage rather than a glial lineage, consistent without our observations. Seigel et al. detected subpopulations of retinoblastoma cells immunoreactive for various embryonic and neural stem cell markers and suggested that the persistence of stem cells may account for the typical early onset(30). In the current study, we found positive correlations between immunoexpression levels of SOX-2 and MAP2 and patient age at the time of enucleation, which supports the idea that retinoblastoma cells are more likely derived from neuronal stem cells in younger patients. While none of the cases showed substantial GFAP immunoreactivity within the tumor, reactive astrocytes showing strong GFAP expression were observed in the surrounding tissue accompanying intra- and peritumoral blood vessels. In contrast, unequivocal areas of reactive gliosis have been described in retinoblastoma, generally in the presence of a disorganized retina. According to Herman et al.(19), the reactive gliosis seen in continuity with retinoblastoma has a Müller cell proliferation pattern. In some cases, tumor growth causes the retina to rise, fold, and become disorganized, while in other tumors, retinal glia can be fully or partially surrounded by the tumor, resulting in the incorporation of isolated mature reactive astrocytes or small groups, as observed in a few of our cases. Our findings suggest that the retinoblastoma "cell of origin" does not express characteristics of mature glial cells or their direct precursors.
Numerous studies have also examined marker expression patterns in various retinoblastoma cell lines. Kyritsis et al.(10) speculated that retinoblastoma originates from a primitive neuroectodermal bi- or pluripotential cell based on findings that the Y79 retinoblastoma cell line expresses NSE and GFAP in the undifferentiated state and one of these two markers in a more differentiated state. A previous comparative study on the immunohistochemical staining pattern of these two retinoblastoma cell lines found positivity for NSE, MAP2, homologous gene class III β tubulin isotype (hβ4), and synaptophysin and negativity for opsin, GFAP, myelin basic protein, myelin-associated glycoprotein, and Leu7. The Y79 line is negative for H neurofilament protein (NFP-H) and S antigen, while the WERI-1 line is positive for both, suggesting that WERI-1 is in a more advanced state of differentiation. Nonetheless, the presence of MAP2 immunoreactivity in both cell lines supports the hypothesis that both arise from mature neuroblasts(19). More recently, Seigel et al. reported the expression of stem cell markers in WERI-1 and Y79. This finding may have important clinical implications because stem cell properties could allow retinoblastoma subpopulations to endure chemotherapy and retain tumor forming potential(30). In the present study, we observed that both Y79 and WERI-1 lines were positive with varying intensity (moderate to intense) for SOX-2 and MAP2 and negative for GFAP, consistent with neuroblast origin. The presence of immature and mature neural markers in these lines corroborates our immunohistochemical findings and again suggests that retinoblastoma may originate from a multipotent neuronal cell lineage that retains some phenotypic heterogeneity.
Two important aspects of this research are (1) the co-application of two neural markers (for immature and mature cells) and a broad spectrum glial marker in a relatively large sample of retinoblastoma cases, and (2) direct comparison with retinoblastoma cell lines. Based on the heterogeneous phenotype of retinoblastoma, it may be necessary to design new treatment protocols using agents able to disrupt proliferation and destroy multiple subpopulations, including hybrid phenotypes.
In conclusion, the immunohistochemical expression of SOX-2 and MAP2 in retinoblastoma indicates a neuroblast/neuronal origin, while GFAP negativity rules out a glial origin. No correlations were found between immunohistochemical expression levels (of SOX-2 and MAP2) and prognostic factors, suggesting these are ubiquitous features of retinoblastoma. The immunocytochemical expression pattern of SOX-2, MAP2, and GFAP in retinoblastoma cell lines (Y79 and WERI-1) further supports the neuroblast/neuronal origin of retinoblastoma.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

