INTRODUCTION
Full-field electroretinogram (ERG) measures the diffuse response of retina cells to a light stimulation. The response is the consequence of light-induced changes in the transretinal movements of ions in the extracellular space, particularly sodium and potassium(1). The electrical activity recorded represents the combination of positive and negative components originated from different stages of retinal processing.
The ERG response is generally expressed as a waveform with negative and positive deflections. The negative deflection, called a-wave, represents the activity of photoreceptors. The positive deflection, called b-wave, represents the ON-bipolar cell function(1). ERG has been used as a diagnostic method for inherited retinal degenerative diseases such as retinitis pigmentosa, congenital stationary night blindness, cone dystrophy, and other conditions(2-4). At times, ERG abnormalities can precede fundus changes(5).
Different types of electrodes can be used for ERG recording, and those that contact the cornea or nearby bulbar conjunctiva during the procedure are most strongly suggested for full-field ERG recording. These electrodes include conductive fibers and foils, bipolar contact lenses, conjunctival loop electrodes, and corneal wicks(6). ERG recording in uncooperative patients and/or patients with palpebral alterations is a challenge because of the difficulty of appropriate placement of the conventional active electrodes. Alternative recording methods such as the use of skin electrodes have been tested to allow ERG recording in this specific group of patients.
The objective of this study was to compare the ERG recorded with skin electrodes and well-established microfiber active electrodes. In addition, normative values for ERG parameters recorded with skin electrodes were determined.
METHODS
This study protocol was evaluated by the Committee of Ethics in Research of the Universidade Federal de São Paulo and was approved in accordance with the principles of the Declaration of Helsinki.
Participants
Healthy volunteers were recruited from university students and employees of Federal University of Sao Paulo and were invited for the full-field ERG test after a full explanation of the procedure, its benefits and risks, followed by written informed consent. The inclusion criteria consisted of normal visual acuity for distance 0.0 logMAR (20/20) or better, lack of visual disorders, normal fundoscopy, and acceptance of the informed consent form. Exclusion criteria consisted of high ametropia (myopia= -4.00 spherical equivalent; hyperopia= +4.00 spherical equivalent), history of previous ocular surgeries, or hereditary eye diseases.
Visual acuity
Presenting visual acuity (with spectacles if used) was measured for each eye using a logMAR chart at 4 m.
Full-field ERG
Full-field ERG was performed in a completely dark room, after maximal pupil dilatation (minimum pupil diameter of 6 mm) with one drop each of 1% tropicamide and 10% phenylephrine hydrochloride, followed by 30 min of dark adaptation.
The Veris electrodiagnostic system (Veris 5.1.9; Electro-Diagnostic Imaging, Sao Mateo, CA, EUA) was used for the ERG recording. Peak-to-peak amplitudes were measured in microvolts (μV), and the implicit time was measured in milliseconds (ms).
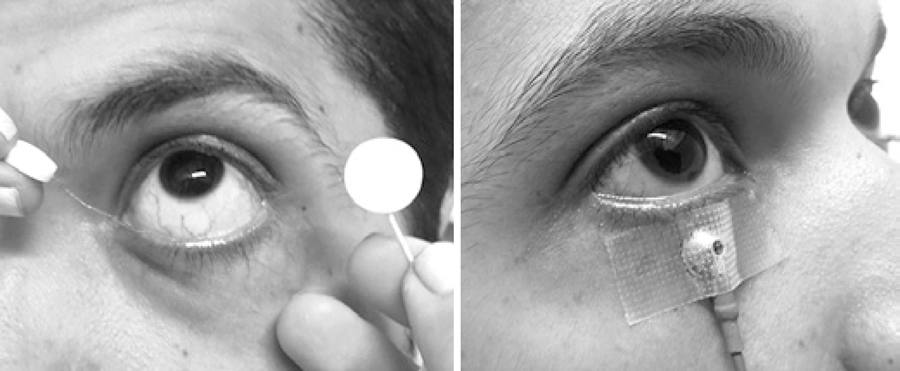
A skin electrode (Grass Gold Disc Electrodes-E5GH) was placed on the lower orbital rim of a randomly chosen eye. On the contralateral eye, an active microfiber electrode was positioned in the lower conjunctival sac (Figure 1). Gold disc electrodes were positioned at the ipsilateral outer canthus of both eyes acting as reference electrodes for the creation of a potential difference. Two ground electrodes were placed on the lobe of each ear.
ERGs were recorded according to the International Society of Clinical Electrophysiology Visual (ISCEV) protocol(7), which is considered the minimum requisite for comparison of different tests performed in different centers(8).
The participants were adapted to complete dark condition for 30 min, and the protocol of ERG recording followed this sequential order: (a) Rod response (a maximum intensity white light with a 2.4 log neutral density filter attenuation); (b) Maximal response (a maximum intensity white light to obtain a combined response of cones and rods after dark-adaptation); (c) Oscillatory potentials (a maximum intensity white light with a 75-Hz low-cut and 500-Hz high-cut filter); and a maximum intensity white light in a 30 cd/m2 background presented at (d) 1 Hz and (e) 30 Hz to obtain cone responses after a 10 min adaptation to a 30 cd/m2 background light (d) single-flash cone response (maximum intensity light at 1 Hz) and (e) 30 Hz flicker response (maximum intensity light at 30 Hz) were recorded.
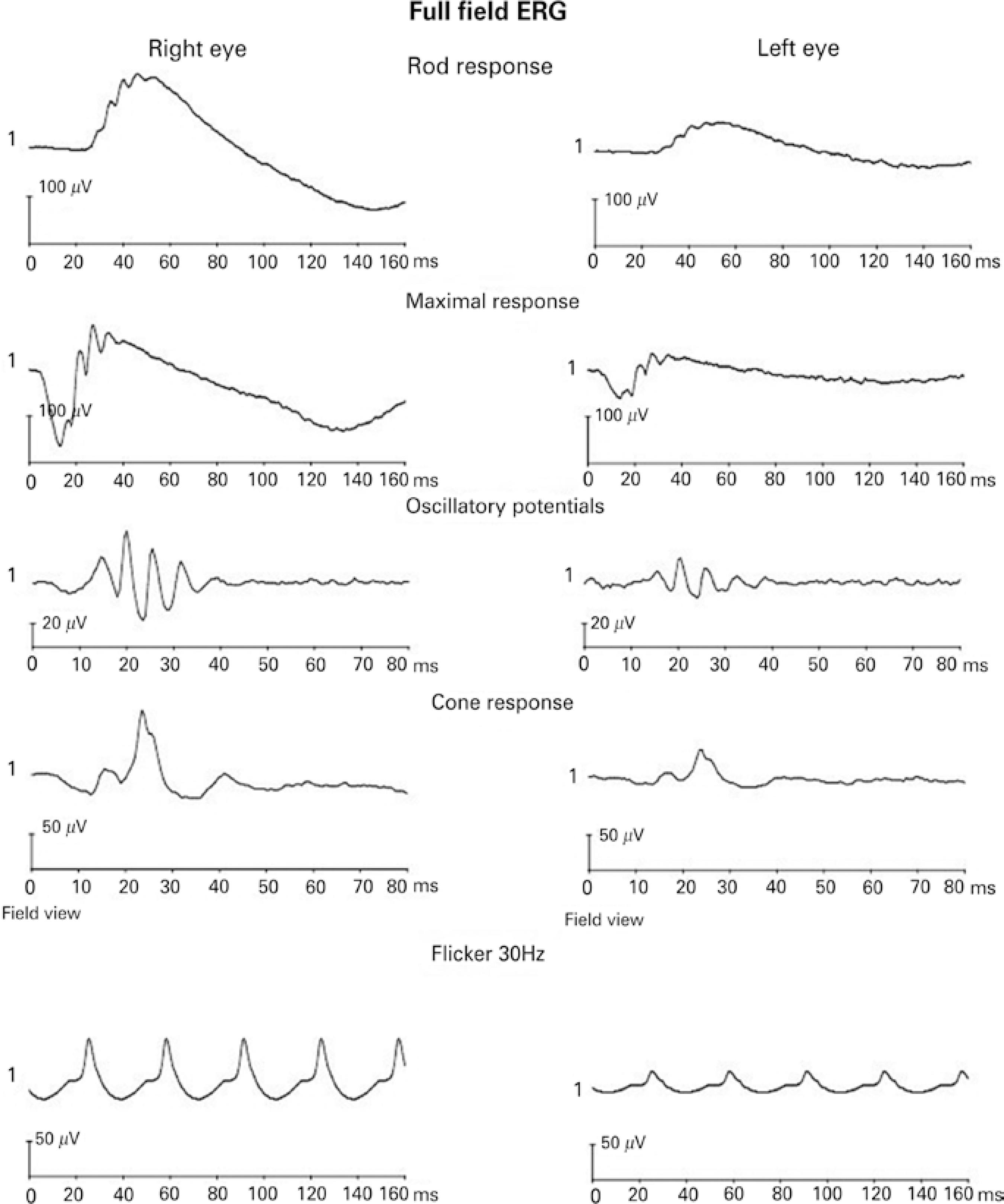
Figure 2 shows a representative full-field ERG recording from a 22-year-old male participant. Right eye responses were recorded with the microfiber electrode, and left eye responses were recorded with the skin electrode.
Statistical analysis
Peak-to-peak amplitude and implicit time from each step of the ISCEV standard ERG protocol were compared between the microfiber electrode and the skin electrode using a statistical package (Jandel Sigmastat-Statistical Software Version 2.0, USA). The significance level used was 0.05. All the parameters were compared between the electrodes using the Mann-Whitney rank sum test. Normative values for skin electrode full-field ERG recordings were calculated as 2.5 and 97.5 percentiles of peak-to-peak amplitude and implicit time.
RESULTS
A total of 50 normal subjects (17-26 years; mean 20.63 ± 2.01 years; 19 males) were recruited for the study based on the inclusion criteria.
Mean amplitudes and implicit times for the five ERG responses are shown in table 1; figure 3 shows the comparison of the recordings of the microfiber electrodes and the skin electrodes. All responses showed similar waveforms for both the electrodes. There was no statistical difference in the implicit time of responses between the two types of electrodes. On amplitude, skin electrode responses showed a significant amplitude reduction (p<0.001) of 61.4% for rod responses, 61.5% for maximal responses, 46.2% for oscillatory potentials, 57.4% for cone responses, and 54.4% for flicker responses, when compared with microfiber electrode responses.
Table 1 Amplitude and implicit time measurements for microfiber electrodes and skin electrodes
|
Peak-to-peak amplitudes of the standardized ERG recorded with microfiber electrodes and skin electrodes | ||
| Microfiber electrode | Skin electrode | |
| Rod response | 198.49 ± 40.30 µV | 81.60 ± 17.88 µV |
| Maximal response | 276.27 ± 48.86 µV | 114.14 ± 22.30 µV |
| Oscillatory potentials | 90.31 ± 49.56 µV | 49.61 ± 32.56 µV |
| Cone response | 108.66 ± 32.02 µV | 47.42 ± 13.36 µV |
| Flicker 30Hz | 50.86 ± 20.73 µV | 24.27 ± 9.00 µV |
| b/a ratio | 1.95 ± 0.42 | 1.76 ± 0.26 |
|
Implicit time of the standardized ERG recorded with microfiber electrodes and skin electrodes | ||
| Microfiber electrode | Skin electrode | |
| Rod response | 55.27 ± 8.97 ms | 53.77 ± 8.54 ms |
| Maximal response | 36.17 ± 6.15 ms | 33.25 ± 5.48 ms |
| Cone response | 25.03 ± 2.77 ms | 24.79 ± 2.59 ms |
| Flicker 30Hz | 27.04 ± 2.42 ms | 26.55 ± 2.73 ms |
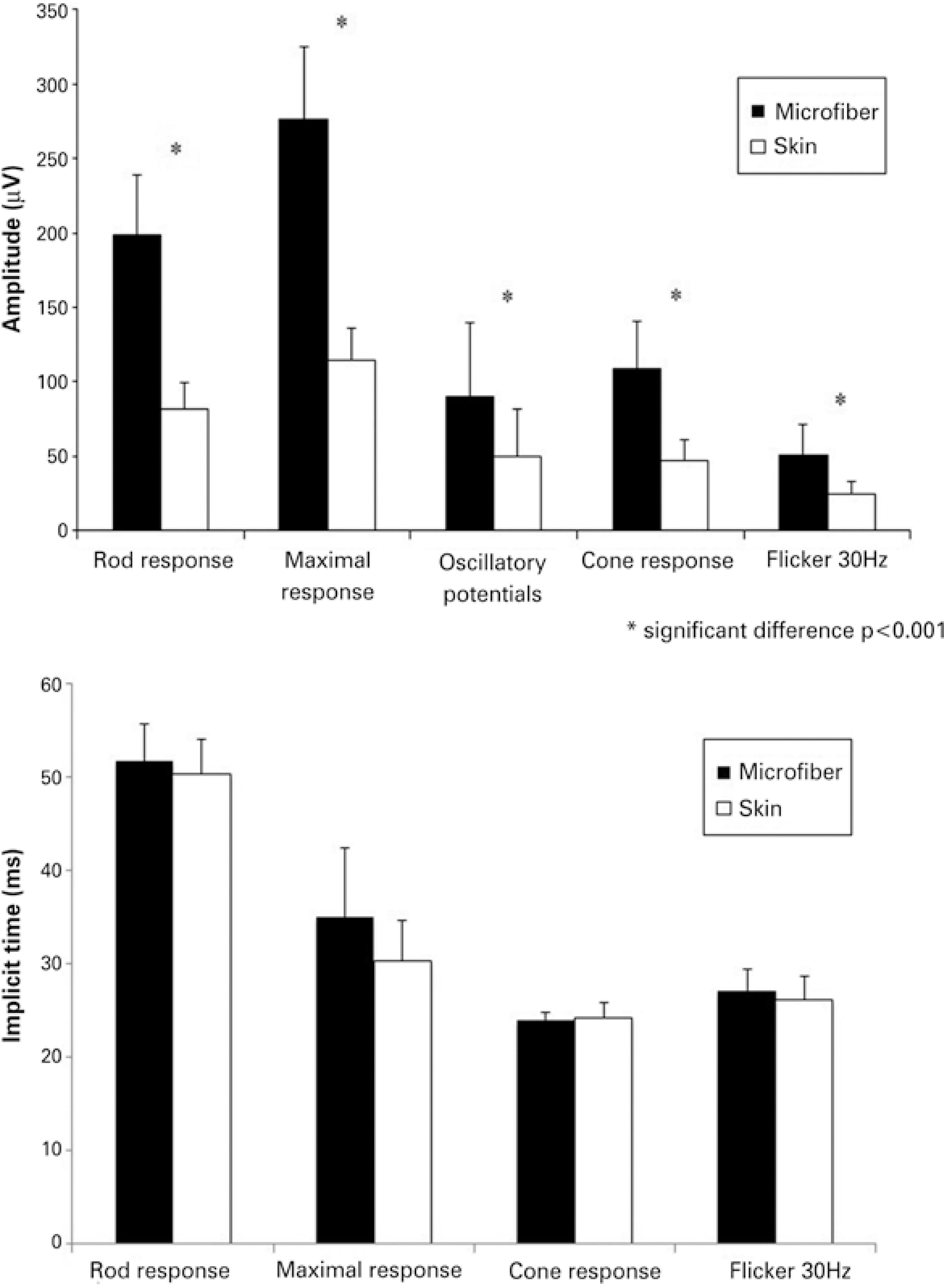
Figure 3 Mean amplitude and implicit time comparison between microfiber electrodes and skin electrodes (*p<0.001). Error bars represent standard deviation of the mean.
Based on these results, normative values for amplitude and implicit time to be used as a reference for full-field ERG recorded with skin electrodes were determined as shown in table 2.
Table 2 Normative values (2.5 and 97.5 percentiles) for full-field ERGs obtained with skin electrodes
|
Normative values for peak-to-peak amplitude for responses recorded with skin electrodes | |
| Rod response | 45.70-112.31 µV |
| Maximal response | 68.82-154.39 µV |
| Oscillatory potentials | 15.05-137.51 µV |
| Cone response | 19.35-74.19 µV |
| Flicker 30 Hz | 11.19-45.56 µV |
| b/a ratio | 1.30-2.31 |
|
Normative values for implicit time for responses recorded with skin electrodes | |
| Rod response | 43.84-75.38 ms |
| Maximal response | 25.50-44.98 ms |
| Cone response | 22.00-30.65 ms |
| Flicker 30 Hz | 24.00-33.69 ms |
DISCUSSION
ERG recordings using skin electrodes produced lower amplitudes and higher electrical noise. This technique is not recommended by ISCEV; however, it can be adopted for specific clinical conditions(7). In this study, it was observed that when using skin electrodes as an alternative method, the responses present similar wave morphology and had no significant difference regarding implicit times for all five ISCEV protocol responses.
The reduction found in peak-to-peak amplitudes recorded with skin electrodes in our study is consistent with previous reports of reductions of 75% and 80%(9), 43%-73%(10), 50%-70%(11), 50%(12), 23%-62%(13), and 42%-57%(14). Our data showed reductions between 46% and 62% on the peak-to-peak amplitudes. Physical factors are involved in this reduction given their influence on the current flow through the electrodes. Such factors include the resistive and capacitative characteristics of the materials from which the electrodes are made and coated with and the area of the recording surface. In addition, the amplitude recorded with skin electrodes is noisier, mainly as a result of electromyographic activity intervention(15). However, it has been shown that even with reduced amplitudes, recordings with skin electrodes can be useful to differentiate between normal and abnormal responses, as long as an appropriate normative reference is used(16). The combination of clinical findings and ERG results demonstrated that ERGs with skin electrodes are accurate for the diagnosis of retinal dystrophies(16).
For pediatric patients, ERG recorded with skin electrodes presents an important advantage because there is no need for sedation. In patients with ocular surface alterations, this technique is suggested as a unique alternative for recording ERG.
Consideration of pediatric age groups could have provided better evaluation of the electrode performance; however, several studies report that peak-to-peak amplitudes of ERG responses are similar in children and adults, irrespective of whether recording is performed with conventional or skin electrodes(17-21). ERG responses are said to achieve adult levels by the fourth year of life; therefore, separate pediatric normative values evaluation would be necessary for children aged <4 years(6).
Previous studies with skin electrodes have demonstrated that peak-to-peak amplitude can show variability as the skin electrode can move during the exam, reducing the amplitudes on the final steps as the electrodes lose adherence to the skin(11). However, comparative studies demonstrated that the variability found with skin electrodes is similar to the variability found with corneal contact electrodes, and examiners need to ensure that the electrode is correctly placed close to the lower orbital rim during the entire exam(9,22).
The ISCEV protocol and the recommendation for each laboratory to determine its own normative values are extremely important for a high quality test. Normative values from different laboratories may differ for several reasons including different equipment, different electrodes, and different settings on the Ganzfeld(23).
Testing using skin electrodes for full-field ERG recording in patients previously diagnosed with retina dystrophy using ERG with conventional electrodes is recommended to allow an evaluation of the validity of the results and accuracy of the skin electrodes as an alternative method.
Our findings are comparable to previous studies, indicating a reduction in amplitude of electroretinography responses recorded with skin electrodes. However, skin electrodes are useful for specific target populations such as uncooperative infants and/or patients with ocular surface alterations. The normative values calculated in this study can be used as a reference for full-field ERG recorded with skin electrodes.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

