INTRODUCTION
Toxic anterior segment syndrome (TASS) is an acute and noninfectious inflammation of the anterior segment. Most cases have been reported as occurring after cataract surgery(1). Anterior segment inflammation usually occurs within 12-48 h after surgery, and the symptoms include decreased visual acuity, increased intraocular pressure, corneal edema, anterior chamber (AC) inflammation, fibrin formation, hypopyon, and fixed pupils. The vitreous body is not infected in this syndrome(2).
Various contaminants, usually from surgical equipment or supplies, including denatured ophthalmic viscosurgical devices (OVDs), preservatives, talc material in surgical gloves, topical ophthalmic ointment, inappropriately reconstituted intraocular preparations, heat-stable endotoxins, and detergents have all been suspected as causes of TASS(1-3). Further, cataract surgery(1-3), iris-supported phakic intraocular lenses (IOLs)(4), penetrating keratoplasty(5), and Descemet's stripping automated endothelial keratoplasty (DSAEK)(6) have all been speculated as causing TASS. Most cases of TASS can be successfully treated with topical steroids and nonsteroidal anti-inflammatory agents(3). To our knowledge, TASS the following DALK case has not been previously reported in the literature.
In this case report, we present a case of TASS following uncomplicated DALK.
CASE REPORT
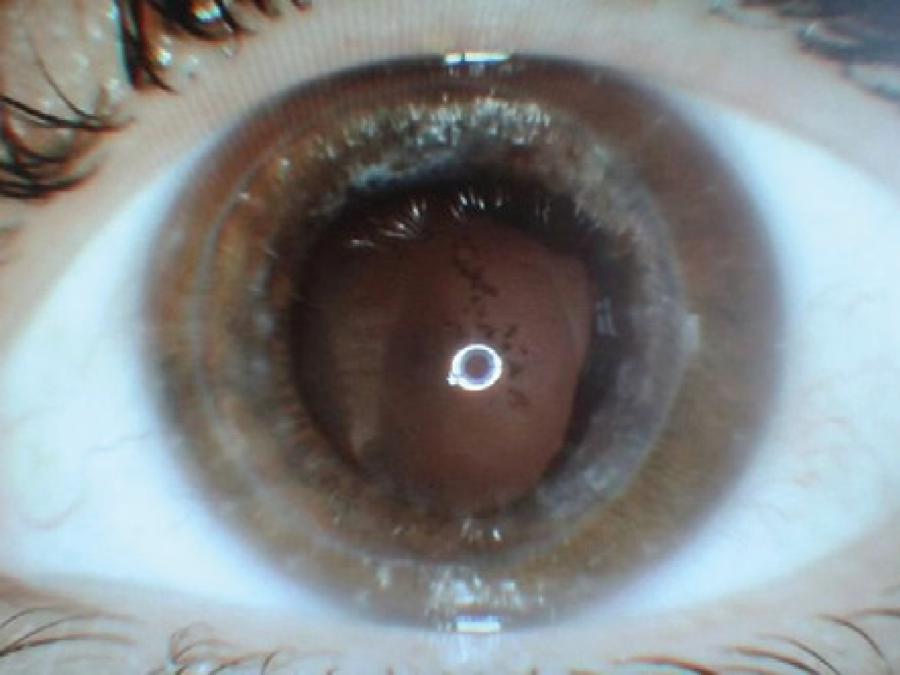
A 32-year-old female patient consulted our clinic due to decreased vision in her left eye. She was being followed for bilateral keratoconus, and she had undergone penetrating keratoplasty in the right eye. On examination, her visual acuity was 20/100 in the right eye and 3-m finger counting in the left eye, with a Snellen chart. The patient had been using a hard contact lens in her left eye; however, no improvement had been seen in her visual acuity, and the patient had to undergo DALK in her left eye. The surgery was performed under general anesthesia, and topical anesthetic drops were not used, although 10% povidone/iodine was used topically at the beginning of the surgery. Descemet's membrane was detached using the "big-bubble" method during surgery. A microvitreoretinal (MVR) knife was used to reduce the AC pressure, and disposable trephine and punch systems were used once to prepare the recipient bed and donor cornea, respectively, and were discarded after use. The donor cornea was sutured to the recipient bed with continuous suture, 360º around, using 10/0 nylon after the endothelium was stripped. Then, the incision site was hydrated with balanced salt solution (BSS), air was let into the AC (the bubble occupied 50% of the AC), and the operation was terminated. The air did not appear to have caused pupillary block. The BSS brand that we used was Ringer's lactate, which had not been used in any other prior surgery. No other drug was topically or intracamerally applied, and moxifloxacin eye drops (0.1 ml) and dexamethasone solution (0.4 ml) were subconjunctivally injected. Ointments were not used at the end of the surgery. Up to this point, we had been using the cannulas that have also been used for viscoelastic injection, a couple of times. To clean them, we routinely wash out the cannulas with distilled water, pass 10 ml of air with an injector through the lumen, and put them in the autoclave immediately after use to avoid waste sedimentation in the lumen. We do not use enzymatic or other cleaning detergents or an ultrasonic bath. During the postoperative course, topical loteprednol etabonate, antibiotic eye drops, and artificial tears drops were initiated. One day postoperatively, the patient had a moderately painful eye; her visual acuity was at the level of hand movements, and there was edema in the cornea. The pupil was mid-dilated, and light reactions were weak. There was no hypopyon in the AC, and no vitreous pathology on ultrasonography. Topical high-dose dexamethasone was initiated, and samples from the solution and donor cornea were sent to a microbiology laboratory. Oral steroids were started because the symptoms did not regress with treatment, and pupillary membrane formation occurred despite treatment (Figure 1 A, B). There were no microorganisms in the cultures. At the follow-up visits, visual acuity increased, and the corneal edema and membrane formation decreased. Treatment was gradually discontinued. At the 18-month follow up, spectacle-corrected visual acuity was 4/10 with a Snellen chart, the cornea was clear, the pupil was mid-dilated, and iris pigments were present on the crystalline lens (Figure 2).
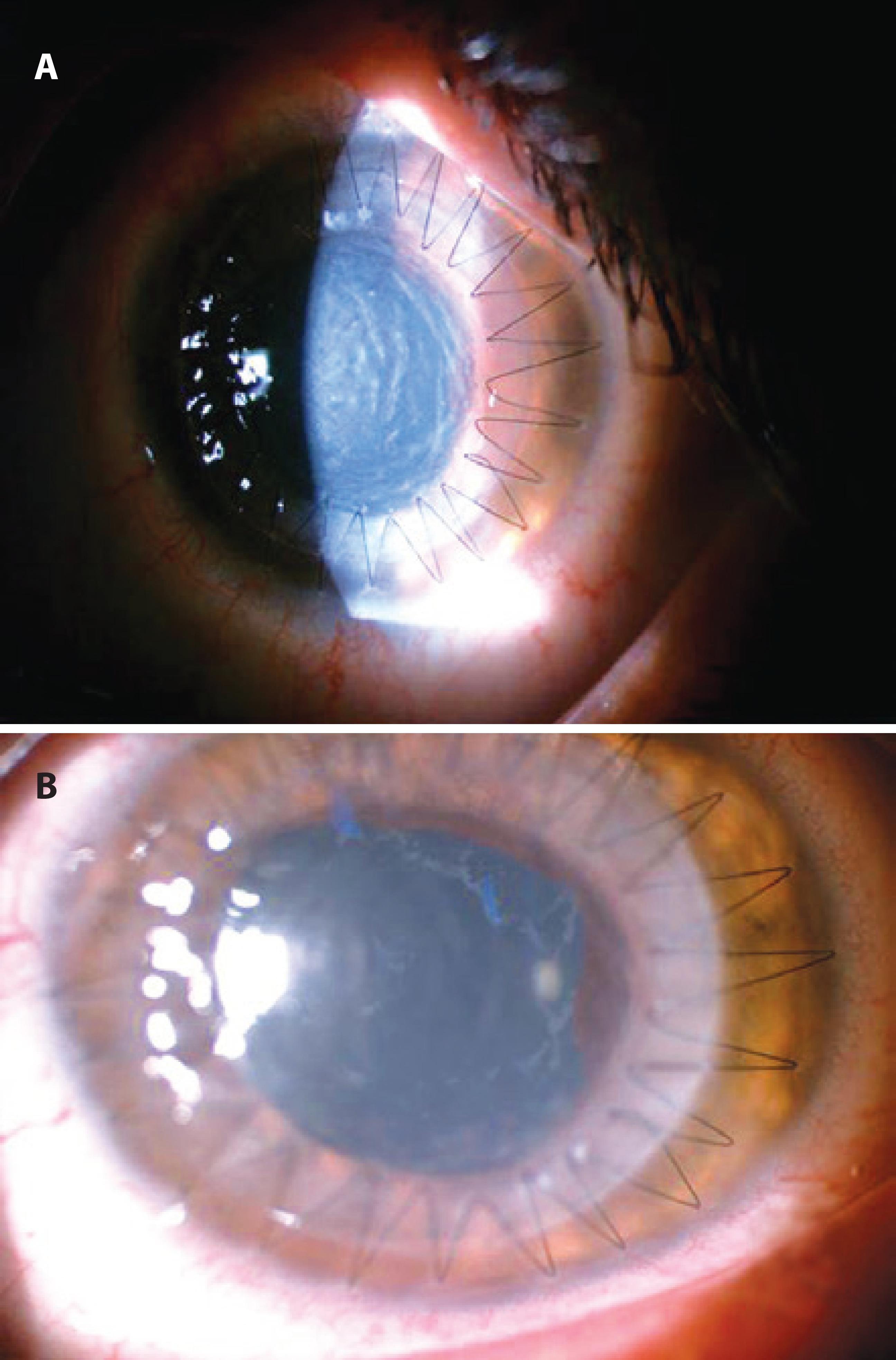
Figure 1 A) Severe postoperative corneal edema; B) 5 days postoperatively, the corneal edema and pupillary membrane formation decreased.
We investigated the medical records and found that there had been no other cases of TASS at our institute over the same period (1 month before and after). In fact, we have not encountered more than three cases of TASS over the last year.
DISCUSSION
TASS is an acute inflammation in the AC, and increasing rates of TASS diagnosis have recently been found(1-3). It is important to differentiate TASS from postoperative endophthalmitis(7). Clinically, TASS occurs during the early postoperative period, with symptoms usually beginning within 12-48 h after surgery(1-3,7). However, it has been reported that early-onset endophthalmitis cases may be seen due to the presence of Staphylococcus epidermidis and Bacillus cereus similarly, which are also seen in TASS(3). Inflammation is restricted to the AC in TASS, and AC reaction, fibrin formation, hypopyon, and corneal edema are observed in this syndrome(8).
TASS often occurs due to contamination of the surgical instruments used during surgery, OVDs, improper intraoperative drugs, or talc material in gloves(1-3,7). The most widely known risk factor is the procedures applied during sterilization(9). Maier et al. detected sterile keratitis in 24 patients following penetrating keratoplasty during the postoperative period. TASS was diagnosed, and they reported that the trephine system was responsible for the development of TASS(5).
In a previous study, TASS was detected in three patients with foldable iris-fixated phakic IOLs. These patients were successfully treated with intensive topical steroids. They also reported that endotoxins in OVDs may cause TASS(4). Sorkin and Varssano reported a case of TASS following DSAEK, phacoemulsification, and IOL implantation. The patient was successfully treated with cycloplegic and topical steroids(6).
There have been reports of TASS following penetrating keratoplasty(5) and DSAEK(6) in the literature, but there has never been a reported case of TASS following DALK. In our case, TASS occurred following uncomplicated DALK. In contrast to penetrating keratoplasty and DSAEK, entry into the AC is extremely rare in DALK. The purpose of entering the AC is to open a side-port with an MVR knife to reduce the AC pressure, thus allowing the side-port to be hydrated with BSS solution and letting some air into the AC. In our case, we used disposable MVR blades. Therefore, we do not believe that TASS occurred from the first incision. We used a re-sterilized cannula for hydrating the incision with BSS and letting air into the AC, and we believe that this cannula was the cause of the development of TASS. The small amount of liquid used to inflate the incision can escape into the AC. If a cannula is used without sterilization or is not cleaned with plenty of fluids, particles remaining in the cannula could cause TASS. The same situation applies during the process of letting air into the AC. These processes are the final stages of the operation, so if any particles enter the AC during these processes, they will not be removed, and may lead to a reaction. TASS is mostly reported after cataract surgery(1-3). There is plenty of irrigation from phaco equipment during phacoemulsification, and more fluid is used during this surgery. Therefore, even if there are particles in the cannula, they may be cleaned or diluted with more liquid, thus lowering the risk of a reaction.
Contact with the AC is relatively lesser during DALK than during other similar procedures. This contact is during the final stages of the operation, and thus, it is important to use a new cannula, or if it is absolutely necessary to re-use it, it should be cleaned by passing plenty of fluids through it.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

