INTRODUCTION
Polypoidal choroidal vasculopathy (PCV) is a disorder that is characterized by dilatation of the choroidal vessels. This disorder was initially reported as idiopathic PCV in 1990(1). Angioid streaks (AS) are breaks in the Bruch's membrane that result in irregular radial or concentric lines around the optic disc; these are mostly associated with pseudoxanthoma elasticum (PXE)(2). In literature, PCV and AS are rarely described in the same patient(3-5), and visual impairment usually occurs because of complications, such as choroidal neovascularization (CNV) during the natural course of both diseases(2,5). Visual acuity can remain unaffected if hemorrhage, subretinal or intraretinal exudation, or pigment epithelial detachments do not develop. Herein we present a case of silent PCV in a patient with AS.
CASE REPORT
A 26-year-old woman was admitted to our clinic for routine ophthalmic examination. Her past ocular and medical histories were unremarkable. At presentation, visual acuities were 20/20 for both eyes, and anterior segment findings for both eyes were normal. Fundoscopy disclosed AS radiating from the optic disc and bilateral changes in the retinal pigment epithelium (RPE) that were evident on the nasal side of the fovea of the right eye (Figure 1 A, B). Optical coherence tomography (OCT) showed a steep, dome-shaped RPE elevation with moderate hyperreflectivity beneath the RPE (Figure 1 C). Due to the suspicion of PCV, we performed fluorescein angiography (FA) and indocyanine green angiography (ICGA). FA demonstrated irregular hyperfluorescence around the optic disc associated with AS in both eyes and increasing hyperfluorescent foci in the nasal side of the fovea of the right eye (Figure 1 D). ICGA showed a focal area of hyperfluorescence surrounded by a hypofluorescent halo in the right macula (Figure 1 E). Neovascularization was excluded by the imaging modalities, and no associated systemic conditions related to AS were found on consultation.
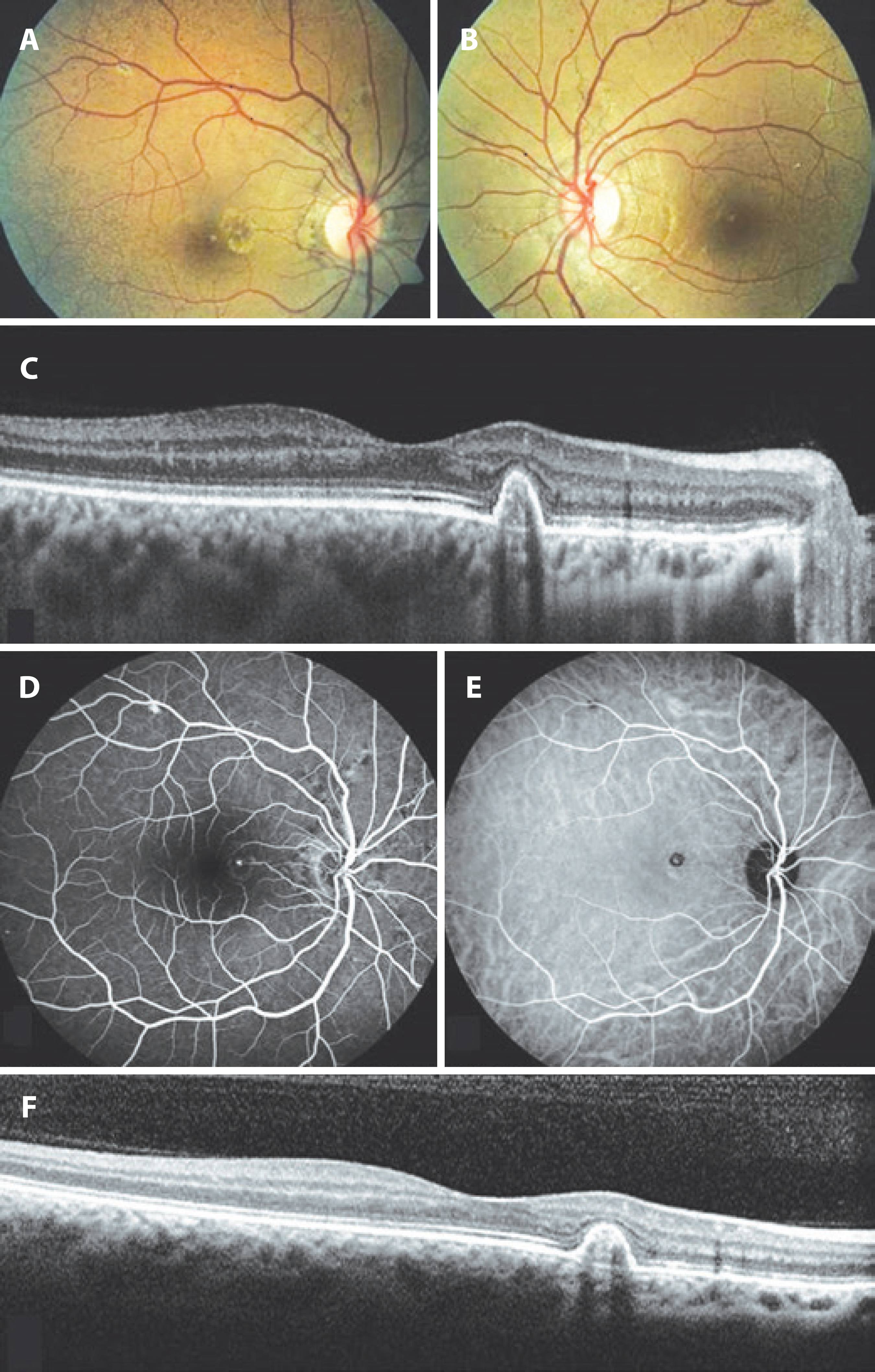
Figure 1 A) Color fundus photography of the right eye demonstrated radial and circumferential angioid streaks (AS) around the optic disc as well as pigment epithelial changes at the nasal side of the fovea. B) Color fundus photography of the left eye showed AS around the optic disc. C) Optical cohorence tomography (OCT) scan through the retinal pigment epithelium (RPE) changes in the right macula at the initial visit demonstrated sharply elevated RPE with moderate hyperreflectivity under it. D) Fluorescein angiography showed dye leakage nasal to the macula in the right fundus. E) Indocyanine green angiography showed a foci of hyperfluorescence surrounded by a hypofluorescent halo in the right macula. F) OCT scan of the same area after 2 years of follow-up showed a polypoidal lesion.
With these findings, we diagnosed the patient with AS in both eyes and PCV in the right eye. Because she had no symptoms and no intraretinal or subretinal fluid was seen on imaging (Figure 1 F), our initial plan was to monitor the patient. The PCV remained silent with no leakage, and no decrease in vision was seen during the 2 years of follow-up.
DISCUSSION
PCV is a localized enlargement of the choroidal vasculature that forms polyps and originates from the inner choroid(1). Hyalinization of vessels as well as plasma and/or fibrin exudation are the histopathological features of PCV(6). Serous or hemorrhagic pigment epithelial detachments, subretinal hemorrhages, and exudation are the common signs of PCV, and these secondary complications cause visual disturbances(6). If the patient has no symptoms and there are no ophthalmoscopic signs, imaging modalities, especially ICGA, can assist physicians in diagnosing the lesion.
PCV has been shown to be associated with pathologies such as tilted disc, high myopia, retinitis pigmentosa, central serous chorioretinopathy, and AS(7-9).
Few cases with coexistence of AS and PCV have been reported in literature(3-5). PCV has been detected at initial examination in some of the cases in literature, and some have developed PCV as observed during the follow-up of CNV due to AS(3-5). The first reported case was initially treated for CNV as a complication of AS in both eyes, and the patient developed PCV temporally to the macula and in the nasal side of the fovea of the right eye during the follow-up period(3).
In a series of 44 cases with AS, PCV was found in six eyes of five patients(4). PCV was identified in two eyes at initial examination, and polypoidal lesions were detected on follow-up visits in four eyes. A patient who was initially diagnosed with PCV developed CNV during the follow-up and was treated with intravitreal SF6 injection, photodynamic therapy, and intravitreal anti-VEGF injection. PCV was identified at the edge of type 2 CNV in two eyes during the follow-up visits. As the lesions were far away from the fovea, they did not require any treatment, which suggests that vascular anomalies causing PCV can be initially detected in patients with AS. Further, some patients may have a predisposition for developing polypoidal lesions.
A 59-year-old male patient with a history of PXE and CNV due to AS developed PCV 1 year after the diagnosis of neovascularization; the patient had already undergone nine intravitreal anti-VEGF injections for CNV(5).
AS is usually related to a systemic condition, with PXE being the most common. Smooth muscle cells play a role in the systemic pathological changes of PXE(10). Abnormalities in the smooth muscle cells of the choroidal vascular structure lead to dilatations that form PCV, and these two entities can show similarities in their pathogenesis(3). Moreover, alterations of Bruch's membrane, which simplify the development of CNV in patients with AS, may also facilitate complications due to polyps. It is also important to keep in mind that there were no associated systemic diseases in the present case, but the patient is still young and there is a possibility that she may develop systemic findings in future. The reported PCV cases in patients with AS have been diagnosed at older ages(3-5). In addition to the pathology of Bruch's membrane in AS, which usually progresses over many years, aging may contribute to the impairment of Bruch's membrane; therefore, complications may easily occur.
PCV lesions can stay silent and do not affect visual acuity if there are no signs of leakage or hemorrhage from the polypoidal lesions(6). Our patient did not report visual loss, and her PCV was diagnosed incidentally. Ophthalmoscopic examination and imaging modalities confirmed the diagnosis of PCV, and she did not develop any complications during the 2 years of follow-up.
In conclusion, patients with AS should be followed-up not only for the development of CNV but should also be followed-up routinely for polypoidal lesions. Vision-threatening complications can occur during the natural course of PCV, and imaging techniques such as ICGA and OCT are the most important tools for the diagnosis of this rare association.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

