INTRODUCTION
Pre-eclampsia is a disorder characterized by widespread vasospasm and pathologic lesions in multiple organ systems, including the uteroplacental vascular bed. Observed in 5%-10% of all pregnancies, pre-eclampsia is a major cause of both maternal and fetal mortality and morbidity. It is typically seen after week 20 of gestation and frequently in first pregnancies(1). In the pathophysiology of pre-eclampsia, although endothelial cell damage and impaired endothelial cell function play an important role, the cause of vascular endothelial cell dysfunction remains unknown(2). Numerous organs, including the liver, eye, kidney, and those of the central nervous system, can be affected by pre-eclampsia due to increased systemic blood pressure and vascular endothelial damage(3). Visual loss was reported in 30%-100% of patients with pre-eclampsia and associated with disorders of the choroidal and retinal circulation(4,5). The presentation of the disorder involves lesions caused by ischemia in the retina pigment epithelium (RPE) and the choroid, as well as cotton wool spots, retinal hemorrhage and edema, papilledema, serous retinal detachment, and Elschnig spots(6).
As such, assessment of the choroid is crucial in ophthalmologic practice for the management of pre-eclampsia, which primarily affects the vascular system. Although indocyanine green angiography and B-scan ultrasonography are usually performed in the clinical assessment of the choroid, neither permits accurate cross-sectional imaging. Consequently, the recently developed optical coherence tomography (OCT) has proven valuable for observing changes in the retina and RPE. Furthermore, a new modality in choroidal imaging with OCT called enhanced depth imaging (EDI) has recently been increasingly used, enabling the acquisition of detailed images of tissues behind the RPE.
This study aimed to investigate subfoveal choroidal thickness (SFCT) in patients with pre-eclampsia during pregnancy and the postpartum period compared with that in healthy pregnant women using EDI-OCT.
METHODS
Study population and design
This cross-sectional study was conducted in the Ophthalmology and Gynecology Departments at Kayseri Education and Research Hospital in Kayseri, Turkey. The study adhered to the tenets of the Declaration of Helsinki and was approved by the local ethics committee. All participants received oral and written information about the study, and each participant provided written, informed consent.
A sample of 73 pregnant women was examined over a period of 28 weeks during gestation. Gestational age was based on the precise date of the woman's last period and ultrasound measurement of the crown-rump length during the first trimester. Pre-eclampsia was defined as hypertension (blood pressure of ≥140/90 on at least two occasions >4 h apart after 20 weeks of gestation) and new onset of proteinuria (≥2 + on dipstick reading, ≥0.3 g/d by 24-h urine collection, or ≥30 mg/mmol by protein-to-creatinine ratio). The study involved two groups: one of pre-eclamptic pregnant women (n=32) and another of healthy pregnant women (n=41). In cases in which both eyes of the patient were eligible for inclusion, only the right eye was included.
Examination protocol and study measurements
Each participant received a comprehensive ophthalmologic examination involving best-corrected visual acuity (BCVA), slit lamp biomicroscopy, dilated stereoscopic fundus examination, intraocular pressure (IOP) measured using Goldmann applanation tonometry, as well as axial length (AL) and OCT measurements. All examinations were performed by an experienced clinician (MU). AL was measured using IOL Master 500 (Carl Zeiss Meditec Inc, Jena, Germany). To assess SFCT, OCT (version 5.6.3.0; Spectralis OCT Heidelberg Engineering, Dossenheim, Germany) was used. All measurements were repeated at the third month of the postpartum period. Moreover, to avoid diurnal fluctuation, all examinations were performed between 10 a.m. and 12 a.m.
Exclusion criteria
Ocular exclusion criteria for this study included more than two diopters of cylindrical and/or spherical refractive error, a BCVA of less than 20/20, IOP readings >21 mmHg, glaucoma, uveitis, retinal disease, ocular trauma, dense media opacities, or a history of previous intraocular surgery, laser therapy, or significant ocular disease. Participants reporting a history of any medical or obstetrical problems (except pre-eclampsia) were excluded from the study.
EDI-OCT measurement
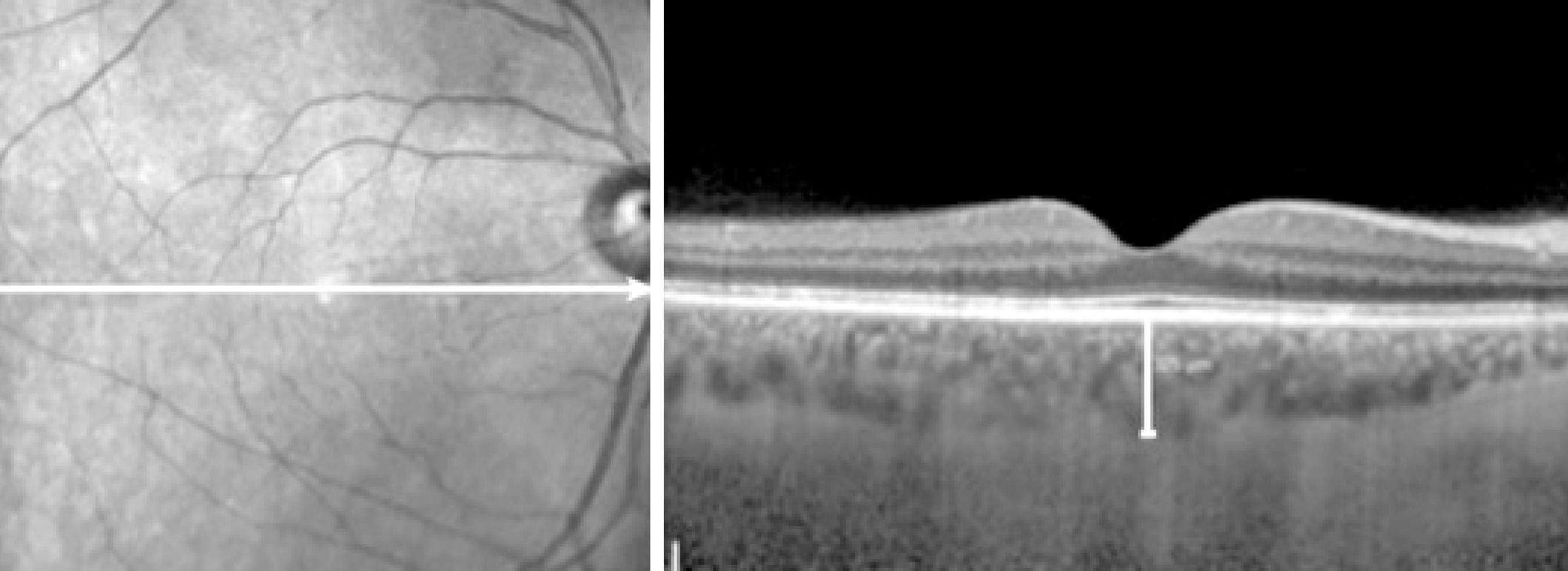
The method used for obtaining EDI-OCT images was previously reported(7,8). SFCT was measured from the outer portion of the hyper-reflective line corresponding to the RPE to the inner surface of the sclera. Images were acquired by an experienced clinician (MU) and assessed by an experienced clinician (MA), the later was blinded to group identity. A representative EDI-OCT choroidal image in a pregnant woman is seen in figure 1.
Statistical analysis
All statistical tests were performed using the Statistical Package for the Social Sciences v.20. Measurements of ocular parameters from the right eyes were used for analyses. For each continuous variable, normality was checked using the Kolmogorov-Smirnov test. Variables between pre-eclamptic pregnant and healthy pregnant women were compared using an independent Student's t test, and a paired t test was used to compare variables between the pregnancy and postpartum periods in each group. Probability (p) values of <0.05 were considered statistically significant.
RESULTS
Table 1 summarizes the demographical and clinical features of the participants. No statistically significant differences were detected in terms of gestational week, age, AL, or IOP between groups (p=0.836, p=0.106, p=0.726, and p=0.936, respectively).
Table 1 Demographical and clinical features of healthy and pre-eclamptic pregnant women included in the study
| Variables | Healthy pregnant women (control group) | Pre-eclamptic pregnant women (study group) | p* |
|---|---|---|---|
| Number of eyes/patients | 41/41 | 32/32 | |
| Age (year) | 0.106 | ||
| Average ± SD | 27.54 ± 5.25 | 29.59 ± 5.43 | |
| Range | 18-38 | 18-43 | |
| Gestational week | 0.836 | ||
| Average ± SD | 31.98 ± 3.82 | 31.81 ± 2.89 | |
| Range | 28-39 | 28-37 | |
| Axial length (mm) | 0.726 | ||
| Average ± SD | 23.04 ± 0.77 | 23.11 ± 0.90 | |
| Range | 21.13-24.16 | 21.34-24.67 | |
| Intraocular pressure (mmHg) | 0.936 | ||
| Average ± SD | 13.37 ± 2.79 | 13.31 ± 2.87 | |
| Range | 9-19 | 9-21 |
SD= standard deviation;
*= independent Student’s t test.
SFCT measurements and p values are shown in table 2. SFCT in pre-eclamptic pregnant women was 351.97 ± 22.44 µm (range, 309-403) and 332.28 ± 20.32 µm (range, 301-381) during the pregnancy and postpartum periods, respectively. In healthy pregnant women, these values were 389.73 ± 49.64 µm (range, 320-473) and 329.78 ± 22.36 µm (range, 299-399), respectively. A significant decrease in SFCT during the postpartum period compared with that during the pregnancy period was found in both groups (p<0.001), but the decrease in choroidal thickness (CT) was more prominent in healthy pregnant women. SFCT in pre-eclamptic pregnant women during pregnancy was significantly thinner than that in healthy pregnant women (p<0.001). However, there was no statistically significant difference in SFCT between the two groups during the postpartum period (p=0.623).
Table 2 The choroidal thicknesses in healthy and pre-eclamptic pregnant women at pre- and postpartum time points
| Prepartum (3rd trimester) |
Postpartum (3rd month) |
p† | |
|---|---|---|---|
|
Healthy pregnant women (SFCT) (µm) N=41 eyes | |||
| Average ± SD | 389.73 ± 49.64 | 329.78 ± 22.36 | <0.001 |
| Range | 320-473 | 299-399 | |
|
Pre-eclamptic pregnant women (SFCT) (µm) N=32 eyes | |||
| Average ± SD | 351.97 ± 22.44 | 332.28 ± 20.32 | <0.001 |
| Range | 309-403 | 301-381 | |
| p* | <0.001 | 0.623 | |
†SFCT= subfoveal choroidal thickness; SD= standard deviation; = paired t test;
*= independent Student’s t test.
DISCUSSION
Although described as hypertension and proteinuria emerging during the second half of pregnancy, pre-eclampsia is a systemic, complex syndrome involving all body systems, not merely reflected by proteinuria and hypertension. Although its etiology is not entirely known, it is thought to consist of common endothelial damage, increased systemic vascular resistance, and generalized vasospasm caused by inadequate trophoblastic invasion and placentation(9,10).
Traditional methods used for assessing ocular findings in pre-eclampsia have, until recently, included indocyanine green angiography, ocular fluorophotometry and angiography, and Doppler ultrasonography(11-16). Low image resolution, low validity ratio in measurements, and differences between observers are disadvantages of these methods. For these reasons, objective, reliable, quantitative, and highly sensitive imaging methods are required for the diagnosis and follow-up of ophthalmic disease in pregnant women. Appropriate imaging technologies were recently developed, one of which is OCT with a high-resolution tomographic section of the optic nerve head, retinal nerve fiber layer, and retina by using 800-840 nm wavelength light in a non-contact, non-invasive manner. More recently, EDI-OCT was developed for users to observe the morphology, thickness, and anatomy of the choroid.
A structurally and functionally normal choroid is critical to retinal functions. Oxygen and glucose are provided to the RPE and outer layers of the retina by choroidal circulation. The choroid also protects the thermal stability of the ocular tissues and removes ocular waste(17). The structure and thickness of the choroid can be affected by several factors, different ocular pathologies, and systemic diseases(18-22). Ocular pathologies such as choroidal neovascular membrane, uveal effusion syndrome, central serous chorioretinopathy, Vogt-Koyanagi-Harada disease, angioid streaks, and polypoidal choroidal vasculopathy, as well as systemic diseases, including diabetes mellitus, can also affect the choroid(18-22). Other studies have reported decreased CT in correlation with increased refractive errors, AL(23-25), or age(26). Several studies have shown the effect of pregnancy on CT. Takahashi et al. compared CT in 30 healthy and 30 pregnant women and found no statistically significant difference between the groups(27). Kara et al. found SFCT to be significantly increased in pregnant women than in healthy women in their study of 100 pregnant and 100 healthy non-pregnant women. They attributed this result to physiological hemodynamic changes occurring in pregnancy, such as increased cardiac output, arterial compliance, and decreased total vascular resistance(28). Meanwhile, Sayin et al. found CT to be decreased in pre-eclamptic pregnant women than in healthy pregnant women(29). Garg et al. assessed CT during the postpartum period in their study of 15 pre-eclamptic pregnant, 15 healthy pregnant, and 19 healthy non-pregnant women. They found that CT was significantly higher in pre-eclamptic women than in the other groups; a result that they attributed to increased vascular endothelial growth(30). Nevertheless, until now, no study has compared CT in pre-eclamptic pregnant women between the pregnancy and postpartum periods. As such, to the best of our knowledge, the present study is the first to assess SFCT in pre-eclamptic pregnant women during and after pregnancy.
In this study, SFCT was found to be significantly thicker in healthy pregnant women than in pre-eclamptic pregnant women. CT decreased by a statistically significant margin, and both groups had similar values by the third month of the postpartum period. As such, it is possible that choroidal thinning in the pre-eclamptic group was caused by vascular vasospasm. Vascular vasospasm narrows choroidal vascular structures, resulting in decreased choroidal blood supply and, in turn, possible choroidal thinning.
In summary, vasoconstriction and the activation of thrombosis occurring with pre-eclampsia impair choroidal microcirculation by causing endothelial cell dysfunction. As a result, increased permeability and/or impairment of the normal blood-retina barrier may occur. Changes in choroidal structure should, therefore, be remembered in the differential diagnosis of visual loss in pre-eclamptic pregnant women. Future prospective studies with more patients are required, nevertheless, to establish the chorioretinal effects of pre-eclampsia.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

