INTRODUCTION
Diabetic retinopathy is the leading cause of vision loss in the elderly(1). Almost 95% of diabetes-related visual impairment, however, is preventable by early diagnosis and photocoagulation therapy(2). A number of multi-center trials have consistently demonstrated the benefits of photocoagulation in high-risk patients with proliferative diabetic retinopathy (PDR)(3,4).
Panretinal photocoagulation (PRP) is painful, and a substantial number of patients are therefore undertreated and at an increased risk of developing blindness(5). Compared with the widely used conventional laser, new technologies provide more comfortable, less harmful and time-saving treatments because of sub-threshold and multi-shot laser photo coagulators(6,7).
The pattern scan laser (PASCAL; Opti-Medica Corp., Santa Clara, California, USA) is a new generation semi-automatic and multi-shot photocoagulator, which uses either a single or predetermined pattern array with pulse durations as short as 10-30 ms(8,9). Navigated laser photocoagulation (NAVILAS; OD-OS GmbH, Teltow, Germany) is another novel computer-based double-frequency ND:YAG laser photocoagulation system (532 nm), which, apart from offering retina navigation, has similar technical specifications as PASCAL (single or predetermined pattern array, 10-30-ms pulse duration)(10,11). Compared with conventional lasers, both modalities use shorter laser pulses, cause relatively less thermal damage to adjacent retinal tissues and are therefore possible to produce relatively less painful photocoagulation. Whether one of these new laser platforms produce less pain than the other during the panretinal photocoagulation is not extensively investigated.
In this study, we sought to compare pain responses in patients undergoing PRP with either PASCAL or NAVILAS for PDR.
METHODS
Patients
Among patients presenting with visual complaints between June and September 2014, those diagnosed with PDR were enrolled in the study. Patients were randomly assigned to receive either NAVILAS or PASCAL photocoagulation therapy. Ethical committee approval and informed consent from patients were obtained, and the study adhered to the tenets of the Helsinki Declaration.
The inclusion criteria were as follows: being older than 18-years, having a diagnosis of diabetes mellitus type 1 or 2, and presenting with a high risk of PDR. A high risk of PDR was defined as neovascularization of the optic disc, neovascularization associated with vitreous or preretinal hemorrhages (NVEs), or neovascularization greater than one-half of the disk area in size accompanying vitreous or preretinal hemorrhage regardless of NVE location.
Patients with a low risk of PDR, those with poor compliance, and pregnant women were excluded. Additionally, patients with a history of focal/grid photocoagulation or PRP, orbital trauma or surgery; those presenting with inflammatory signs; those with significantly increased corneal or lens thickness; and those with vitreous hemorrhage were also excluded.
All patients were subject to the following assessments: slit-lamp biomicroscopy, intraocular pressure measurement with Goldmann applanation tonometry, fundus fluorescein angiography, and ophthalmologic examination including fundus assessment.
Laser application
Patients were randomly assigned to either the PASCAL (30 patients) or NAVILAS (30 patients) groups. All patients were treated by the same surgeon and underwent a single PRP session. Treatments were performed under topical anesthesia.
The spot size (200-400 µm) and pulse duration (30 ms) used to obtain a white-grayish spot on the retina were identical in both procedures. To objectively compare pain responses, PRP was used in the multi-shot mode and was applied within similar retinal areas, and the total number of spots delivered was equivalent.
Pain perception
After 30 min of PRP application, patients were asked to verbally describe their pain perception as either "none," "mild", "moderate", "severe", or "very severe" through a verbal rating scale (VRS). Additionally, they were asked to specify the severity of pain through a visual analog scale (VAS) by indicating a score from "0" to "10," representing the severity of pain from "no pain" to "severe pain."
Statistical analysis
Statistical analyses were performed using the SPSS (Statistical Package for Social Sciences Inc., Chicago, IL, ABD) version 17.0 software. Data distribution was assessed by the Kolmogorov-Smirnov test. Comparisons were performed using the Mann-Whitney U test and t -test for independent samples.
RESULTS
A total of 60 eyes of 60 patients (20 females and 40 males) diagnosed with PDR were treated. The mean age of patients was 62.22 ± 9.19 years, and the mean duration of diabetes was 195.47 ± 94.54 months. Patient demographics are presented in table 1. There were no significantly differences between the groups in terms of mean age, gender, and mean diabetes duration.
Table 1 Demographic characteristics of study individuals
| NAVILAS | PASCAL | P | |
|---|---|---|---|
| Gender (F/M) | 8/22 | 12/18 | 0.270* |
| Age (mean ± SD) | 63.3 ± 9.4 | 61.1 ± 9.1 | 0.351** |
| Duration of diabetic disease (mean ± SD) | 206.8 ± 101.1 | 184.1 ± 87.8 | 0.480** |
*= chi-square test;
**= independent t-test.
The mean number of laser spots delivered during PRP was 389.47 ± 71.52 in the NAVILAS group and 392.70 ± 54.33 in the PASCAL group. The difference was not significant (p=0.57).
The difference in pain response between patients in the NAVILAS and PASCAL groups was significant with regard to both mean VRS (1.10 ± 0.67 vs.1.47 ± 0.69, respectively; p=0.042) and mean VAS (2.13 ± 1.17vs. 2.97 ± 1.35, respectively; p=0.034) scores (Table 2).
Table 2 Pain scores associated with NAVILAS laser and PASCAL laser treatments
| NAVILAS | PASCAL | P | |
|---|---|---|---|
| Verbal score | 1.10 ± 0.67 | 1.47 ± 0.69 | 0.042 |
| VAS | 2.13 ± 1.17 | 2.97 ± 1.35 | 0.034 |
VAS= visual analog scale; p= Mann-Whitney U test.
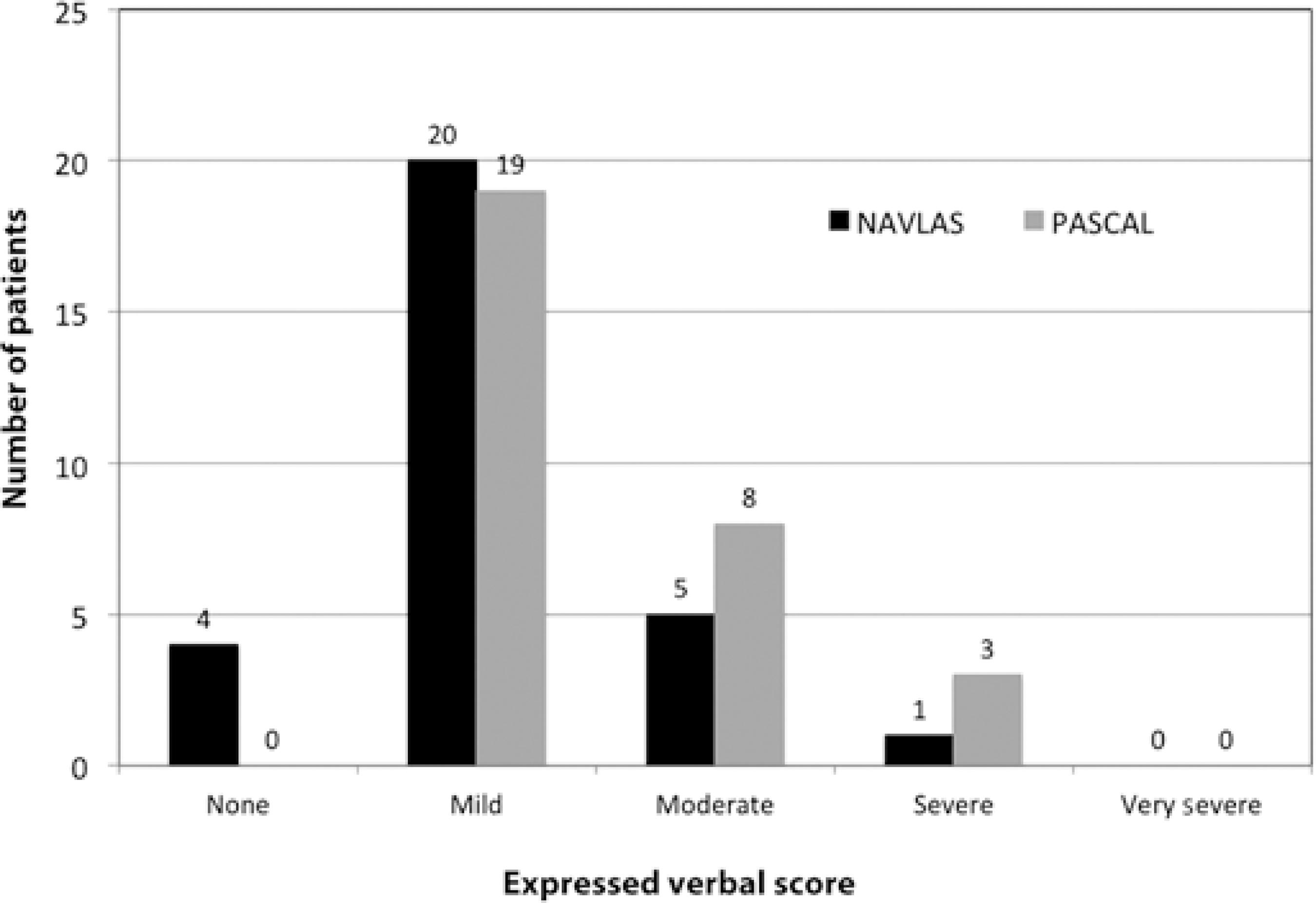
While no patients in the PASCAL group reported "no pain" in the VRS assessment, four (13.3%) in the NAVILAS group reported "no pain" associated with PRP application. A total of 11 of 30 patients reported experiencing moderate or severe pain during PASCAL laser treatment compared with 6 of 30 treated with the NAVILAS laser. None of the patients in either groups reported "very severe pain." Patient distribution according to VRS is presented in figure 1.
DISCUSSION
The advent of photocoagulation in 1967 was a critical step in the treatment of diabetic retinopathy and maculopathy(12). PRP treatment may cause considerable pain and discomfort in some patients and may consequently yield reluctance to continuing treatment sessions, thus leading to visual deterioration. By changing laser parameters, however, it may be possible to improve comfort and reduce pain(13). In the current study, we found that patients undergoing PRP treatment with the NAVILAS system experienced significantly less pain than those treated with the PASCAL system with a pulse duration of 30 ms.
The Diabetic Retinopathy and Early Treatment Diabetic Retinopathy studies, using a single-shot conventional laser device, established the minimum laser power levels in PRP treatment with a spot size of 200-500 mm and a pulse duration of 100-200 ms(14,15). Novel laser treatment systems, however, use the multi-shot mode with short pulse duration, thereby providing shorter, less demanding, and less painful treatment sessions(16). Furthermore, studies have shown that a short pulse duration does not negatively influence PRP treatment efficacy(16-18).
Targeting the retinal pigment epithelium while preserving adjacent photoreceptors through micro-air bubble formation formed around melanosomes is a novel target therapy modality of laser photocoagulation. The critical threshold between thermal and mechanical damage is 50 ms(19-21). Owing to the use of shorter pulses, novel laser therapy techniques cause only mechanical but not thermal damage, limiting the damage to the retinal pigment epithelium and preserving the inner retinal layers and sensory-rich chorioretinal tissues(17). Consequently, patients usually report less pain, most probably because retinal sensitivity is better preserved as compared with pain in conservative approaches(9,17). Recent studies have consistently shown that patients treated with either the PASCAL or NAVILAS laser system modalities experienced less pain as compared with those treated with conventional laser treatment modalities(9,10,17,22).
The reason why patients treated with the NAVILAS system experience less pain than those treated with the PASCAL system may in part be attributed to the fact that the former uses infrared light instead of the bright slit-lamp light used in the latter. Infrared light is known to cause less photostimulation(16).
Additionally, both conventional and PASCAL treatment systems require the tilting and moving of a contact lens for treating the peripheral retina, which may cause discomfort(23). The NAVILAS system, on the contrary, uses a specifically designed contact lens and does not require tilting of the lens for the examination and treatment of the retinal periphery(24). The tilting of the lens is also related to laser focusing, significantly influencing spot size and energy density at the level of the retina(23). Focusing is performed by moving the slit lamp closer or further away from the eye in the PASCAL laser system, where laser and image foci may not be identical. On the other hand, the NAVILAS laser system enables the documentation of focus settings adjusted prior to treatment to achieve a clear focused retinal image and focused laser beam(25). As a recent study revealed, laser spots delivered during NAVILAS photocoagulation are more accurate than those delivered using the PASCAL system. Thus, inadvertent laser application is minimized, collateral damage within the retina is decreased, and the preservation of retinal sensitivity is improved, eventually resulting in more comfort and less pain(24).
Response to pain among individuals may vary depending on factors such as culture, gender, threshold of pain, degree of fundus pigmentation, and history of previous laser treatment(17,26). None of the patients included in this study had a previous history of laser treatment. Additionally, randomization provided a more objective assessment of pain. Nevertheless, these factors may still, in part, account for the difference observed in the perception of pain.
Our review of literature identified two additional trials, both conducted by Chablani et al.(16,24), comparing differences in the perception of pain between patients with PDR undergoing NAVILAS or PASCAL photocoagulation. In both studies, a pulse duration of 100 ms was used, and it was found that treatment-related pain was significantly milder following NAVILAS than following PASCAL photocoagulation(24). In the current study, however, we found that treatment with a pulse duration of 30 ms also resulted in milder pain in patients treated with NAVILAS compared with those treated with PASCAL. This issue has rarely been investigated and merits further consideration with long-term follow-up studies on larger groups.
While the relatively small number of spots delivered may appear to be a limitation, the main purpose of the study was to compare pain responses and not treatment efficacy. In the Diabetic Retinopathy Clinical Research Network (DRCR.net) clinical trial, no clinical difference was found when comparing PRP in one session versus that in four sessions in terms of pain perception(27). One limitation was the small sample size. Additionally, we followed all patients and performed the second and third treatment sessions after one week at the latest and eventually completed PRP treatments within one month at the latest.
To conclude, the new, navigated laser approach provided by the NAVILAS system enables the delivery of multiple laser spots in a relatively shorter time with improved accuracy and efficacy, significantly reducing pain and improving patient compliance.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

