INTRODUCTION
Uveitis is defined as inflammation of the uveal tract and has been shown to be associated with a number intraocular diseases, including cataract(1-3). Experimentally, uveitis can induced by intravitreal lipopolysaccharide (LPS) injection(4,5)leading to inflammatory cell infiltration at four hours after injection, with peak inflammation between 18 and 24 hours later (4). Acute inflammatory responses in uveitis are maintained by the action of molecular mediators which leads to decreased active secretion of aqueous humor and increased vascular permeability(6,7).
Pro-inflammatory cytokines, such as interleukin-1 alpha (IL-1 alpha) and interleukin-1 beta (IL-1 beta), have been shown to actively recruit leukocytes to sites of inflammation (6). IL-1 alpha and IL-1 beta are predominantly secreted by activated macrophages, B lymphocytes, and endothelial cells (8). Tumor necrosis factor-alpha (TNF-α) is also synthesized by monocytes, macrophages, neutrophils, mast cells, and T lymphocytes(9). As inflammation progresses, TNF-α modulates leukocyte infiltration by promoting the synthesis of molecules involved in adhesion, maturation, and maintenance of dendritic cells(9). Evidence suggests that elevated expression of TNF-α, as observed in immune-mediated uveitis, correlates with pea inflammation(10). In corroboration, a recent study reported significantly increased expression of TNF-α at 18 hours after cataract surgery(11).
Prostaglandins (PGs) have also been shown to cotribute to intraocular inflammation(12-14). Infectious disease, trauma, ocular surgery, and neoplasia may also cause injury to the aqueous-blood barrier, leading to increased PG synthesis (7). Ribeiro et al.(13)and Gilmour and Payton (14) reported the utility quantifying prostaglandin E2 (PGE2) levels as a measure of intraocular inflammation in dogs.
Opioids bind to specific endogenous receptors inducing antinociceptive effects in the central nervous system. Studies have demonstrated that local application of opoids can elicit analgesia via the neuroimmune system due to the presence of opioid receptors in peripheral tissues(15,16). Wenk et al. (15) demonstrated attenuation of leukocyte infiltration by infusion of morphine directly into corneal ulcerations in mice. Clark et al. (17)demonstrated the efficacy of parenteral administration of morphine reducing cutaneous expression of IL-1 beta, interleukin-6, and TNF-α, and local neutrophil infiltration. Studies have also demonstrated the expression of kappa opioid receptors in the anterior chamber of the eye(18). In humans and primates, opioids are known to suppress chemotaxis and migration of neutrophils, thereby modulating adaptive immune responses(19). Dortch-Canes and Russell(20) reported that morphine instillation reduced the intraocular pressure in healthy rabbits but only for five minutes.
Naloxone is a non-selective opioid antagonist that may be used to reverse the action of morphine(21). Its effects, however, are limited when administered in patients that have not been pretreated with opioids. The half-life of naloxone is approximately one hour as it is rapidly metabolized by the liver(22).
The stability of the aqueous-blood barrier was evaluated by Ribeiro et al. (16)as the local administration of opioids, particularly morphine, is frequently used for pain control in ulcerative keratitis. Studies have demonstrated that morphine inhibits the release of proinflammatory cytokines in response to skin incisions and decreases the infiltration of leukocytes in silver nitrate-induced corneal ulcers(15,17). In corneas following lamellar keratectomy, morphine reportedly increases the number of extracellular metalloproteinases without altering the expression of interleukin-10(16). Husain et al.(23) observed that intraperitoneal administration of morphine has protective effects on retinal ganglion cells in a rat model of experimental ocular hypertension, most likely though inhibition of TNF-α production. A previous study at our institution demonstrated, with the use of laser flaremetry, that morphine increases the total protein content of the aqueous humor when administered after paracentesis of the anterior chamber(24).
The present study aimed to evaluate the effects of morphine instillation on rabbit eyes with uveitis. Morphine is used to promote analgesia in ulcerative keratitis, and after refractive surgeries, conditions that lead to the breakdown of the aqueous-blood barrier and increased expression of cytokines and other inflammatory mediators.
METHODS
A total of 24 New Zealand White adult rabbits (Oryctolagus cuniculus) with a mean weight of 3.0 kg were used in the present study. Rabbits were kept in clean and sanitized individual cages in a ventilated environment. Food and water were offered ad libitum. Only rabbits free of ocular and systemic diseases were used in the present study.
Intravitreal injections in the right eye of each rabbit were given under dissociative anesthesia (ketamine Agener 10% Agener Uniao, Toronto, ON and xylazine Dopaser, Hertape Calier, Juatuba, MG) following the instillation of anesthetic eye drops (anestésico®, Allergan, Guarulhos, SP). Under aseptic conditions, 0.1 mL of a solution containing LPS (0.2 µg endotoxin from the Salmonella typhimurium cell wall; L6511, Sigma Chemical Co.; St. Louis, MO) was applied approximately 3.5 mm from the sclerocorneal limbus to induce uveitis. A 0.25 × 0.7 mm needle and 1 mL syringe were used as previously described(3). Ophthalmoscopy was performed to confirm the absence of iatrogenic retinal lesions(25).
Eyes were clinically evaluated for conjunctival hyperemia, chemosis, blepharospasm, ocular discharge, and aqueous turbidity(7). Laser flaremetry was used to quantify the turbidity of the aqueous humor (Laser Flaremeter® FC-600, Kowa, Japan) before uveitis induction and at 10 and 20 hours after inflammation induction.
Rabbits were divided into four groups (n=6 each): control (CG), morphine (MG), naloxone (NG), and morphine-naloxone (MNG) groups. Eyes with endotoxin-induced uveitis, in a total of 10 applications, received one drop of 0.9% saline solution (CG), 1% morphine (MG), 1% naloxone (NG), or morphine and naloxone (MNG) immediately after the induction of inflammation and at regular 2-hour intervals. Commerically available ophthalmic solutions of 1% morphine sulfate (experimental 1% morphine ophthalmic solution without preservatives, Cristália, Campinas, SP) and 1% naloxone (experimental 1% naloxone ophthalmic solution without preservatives, Cristália, Campinas, SP) were used with the pH adjusted to 6.0. In the MNG, morphine was administered first followed by naloxone 10 minutes later.
Twenty hours after uveitis induction, rabbits were euthanized (overdose of thiopental sodium, Thiopentax, Cristália, Campinas, SP) and subconjunctival enucleation of the right eye was performed. Immediately after, the iris and ciliary body were collected (Figure 1), stored in tubes, and cryogenically preserved at a temperature of -80°C until further use. Approximately four months later, stored samples were macerated, and TNF-α, IL-1beta, MPO, and PGE2 levels were quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer›s recommendations (Enzyme-linked Immunosorbent Assay kit, Uscn Life Science Inc., China).
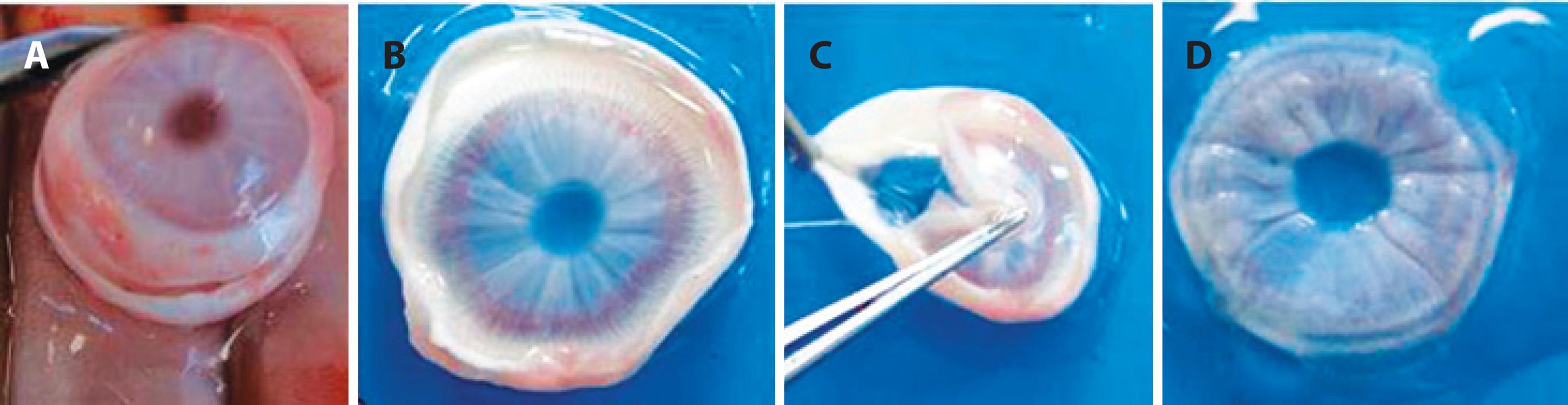
Figure 1 Protocol used following enucleation of the right eye in New Zealand white rabbits. A) scleral section to remove the corneoscleral button; B) corneoscleral button; C) separation of the iris and ciliary body by traction; and D) iris and ciliary body storage conditions.
Nonparametric Friedman analysis of variance with post-hoc analysis using the Dunn's test was used to evaluate aqueous humor flare data, expressed as the median, maximum, and minimum. Data related to cytokine levels were compared using one-way ANOVA followed by Turkey's test. Cytokines values were expressed as means and standard errors of the mean. P values <0.05 were considered statistically significant (SigmaStart 3.0®, Systat Software Inc., San Jose, USA).
RESULTS
Conjunctival hyperemia, chemosis, blepharospasm, ocular discharge, and aqueous turbidity were observed two hours after LPS injection and persisted for 20 hours in all eyes with endotoxin-induced uveitis.
The median aqueous humor photon counts at 20 hours were not significantly higher than those at the baseline in MG (2.10 vs. 4.10 PC/ms), NG (3.80 vs. 4.40 PC/ms), or MNG (2.90 vs. 3.25 PC/ms) (Figure 2). In CG, there was a trend toward increased median photon counts at baseline compared to other time periods; however, this trend did not reach statistical significance. Twenty hours after the induction of uveitis, there was a trend toward a higher median photon in MG (9.20 PC/ms), followed by MNG (7.90 PC/ms), NG (5.00 PC/ms), and CG (4.25 PC/ms); however, this trend did not reach statistical significance.
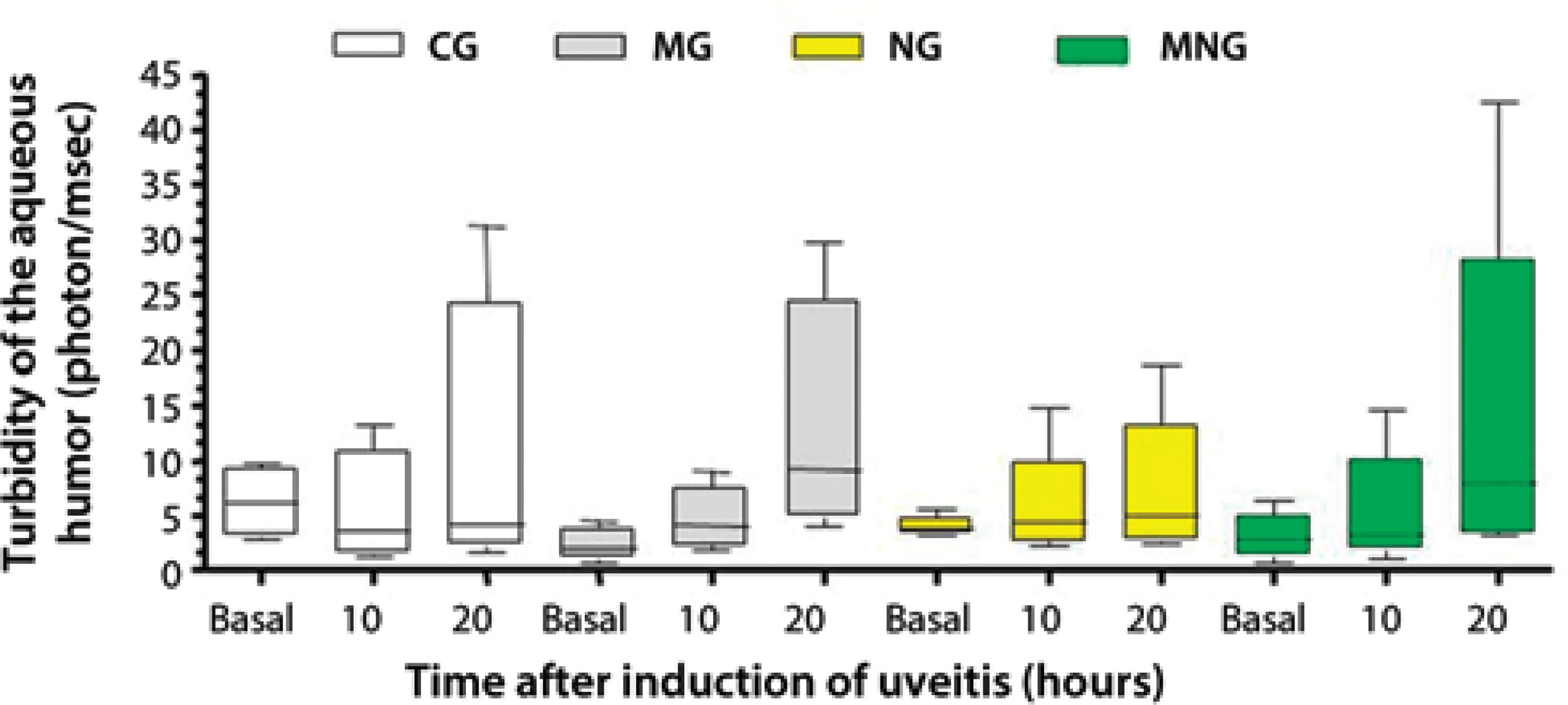
* Dunn test (P>0.05).
Figure 2 Median (lines inside boxes), maximum, minimum, and inter-quartile ranges (25 and 75%) for aqueous humor turbidity (photon/msec) before (basal) and 10 and 20 hours after the induction of uveitis in New Zealand white rabbits divided into the following groups: 0.9% saline (CG), 1% morphine (MG), 1% naloxone (NG), and 1% morphine followed by 1% naloxone (MNG).
In all groups, uveitis induction resulted in increased expression of TNF-α and IL-1 beta (Table 1). Significant differences in TNF-α values as compared to baseline levels (467.1 ± 54.54 pg/mL) were observed in CG (5652 ± 145.7 pg/mL), NG (4601 ± 599.7 pg/mL), MG (5442 ± 417.7 pg/mL), and MNG (4714 ± 426.9 pg/mL; P<0.001 for all). IL-1 beta levels were significantly increased in CG (950.1 ± 71.97 pg/mL), NG (1087 ± 127.9 pg/mL), MG (1137 ± 96.53 pg/mL), and MNG (994.2 ± 86.03 pg/mL) compared to baseline values (509.3 ± 49.59 pg/mL; P<0.001 for all). No significant difference in PGE2 values compared to baseline values (569.7 ± 73.99 pg/mL) were observed in CG (632.1 ± 77.44 pg/mL), NG (663.7 ± 32.67 pg/mL), MG (694.2 ± 45.12 pg/mL), or MNG (449.4 ± 27.29 pg/mL); however, a significant difference was observed between the MG (694.2 ± 45.12 pg/mL) and MNG (449.4 ± 27.29 pg/mL; P<0.001). No significant difference in MPO levels were observed between baseline (4.780* 10-4 ± 1.647* 10-4mU/mL) and treated groups (CG, 7.975* 10-4 ± 2.362* 10-4mU/mL; NG, 8.826* 104- ± 3.697* 10-4 mU/mL; MG, 3.838* 10-4 ± 1.615* 10-4 mU/mL; MNG, 7.086* 10 -4 ± 1.935* 10-4mU/mL).
Table 1 Expression levels of tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1 beta), prostaglandin E2 (PGE2), and myeloperoxidase (MPO), before (Baseline) and after 20 hours of induced uveitis in New Zealand white rabbits, quantified by enzyme-linked immunosorbent assay (ELISA) in the following groups: control (CG), morphine (MG), naloxone (NG), and morphine-naloxone (MNG). Data presented as mean ± standard error of the mean
| TNF-α (pg/mL) | IL-1 beta (pg/mL) | PGE2 (pg/mL) | MPO *10-4 (mU/mL) | |
|---|---|---|---|---|
| Baseline | 467.1 ± 54.54 | 509.3 ± 49.59 | 569.7 ± 73.99 | 4.780 ± 1.647 |
| CG | 5652 ± 145.7* | 950.1 ± 71.97* | 632.1 ± 77.44 | 7.975 ± 2.362 |
| NG | 4601 ± 599.7* | 1087 ± 127.9* | 663.7 ± 32.67 | 8.826 ± 3.697 |
| MG | 5442 ± 417.7* | 1137 ± 96.53* | 694.2 ± 45.12** | 3.838 ± 1.615 |
| MNG | 4714 ± 426.9* | 994.2 ± 86.03* | 449.4 ± 27.29** | 7.086 ± 1.935 |
*= statistically different from baseline;
**= statistically different between groups.
The results presented in figure 3 demonstrate that the observed increases in the expression levels of all cytokines were similar between groups, except for PGE2 values which were lower in the MNG group.
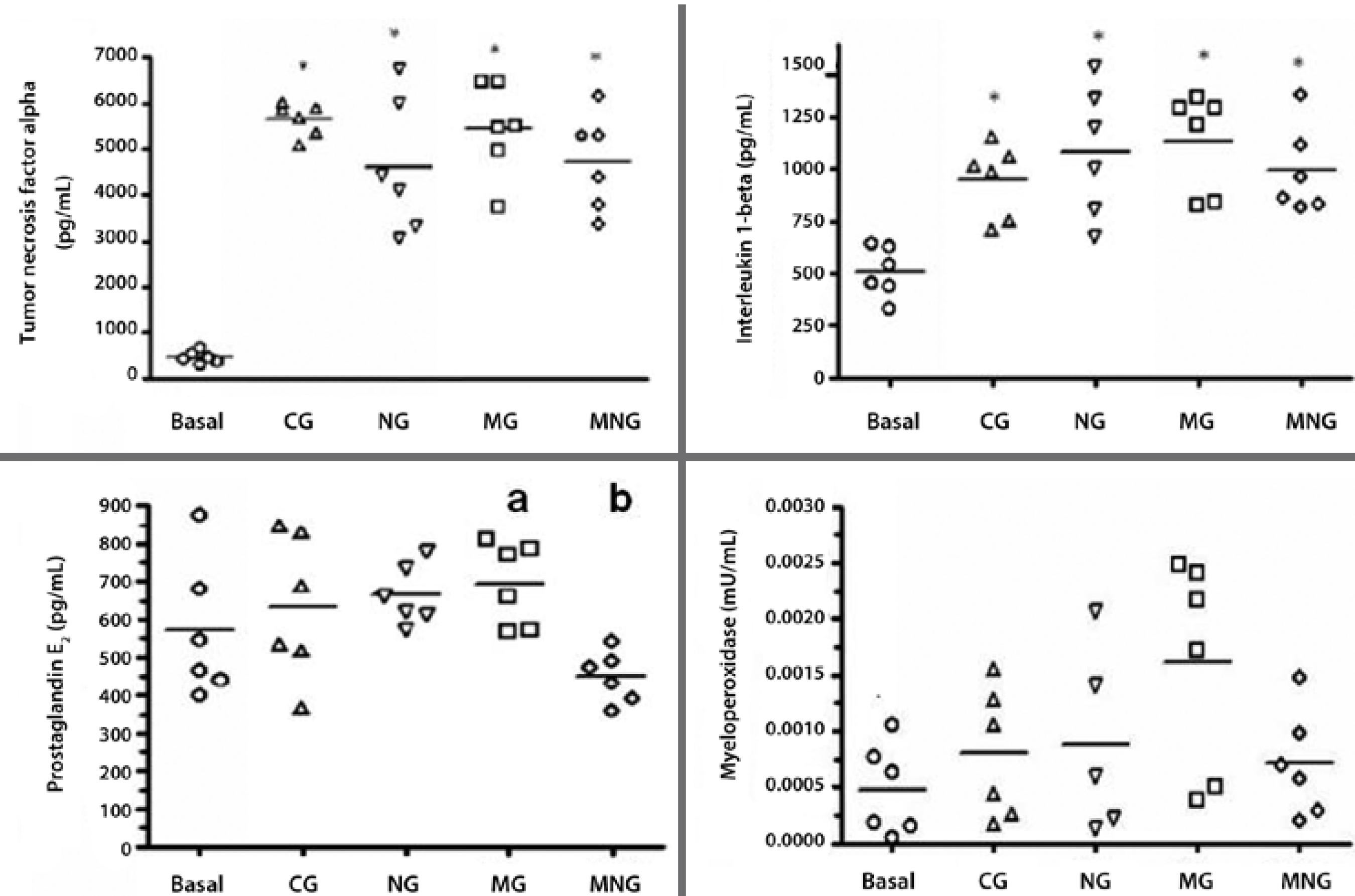
* Turkey's test (P<0.001).
Figure 3 Expression levels of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1 beta), prostaglandin E2 (PGE2), and myeloperoxidase (MPO) before (basal) and after uveitis induction in New Zealand white rabbits divided into the following groups: 0.9% saline group (CG), 1% morphine (MG), 1% naloxone (NG), and 1% morphine plus 1% naloxone (MNG). Horizontal lines represent mean values, geometric symbols () represent data dispersion.
DISCUSSION
LPS injection has been demonstrated to be valid as an experimental model of uveitis and can be performed via intraperitoneal, subcutaneous, intracameral, and intravitreal routes(4,5). The intravitreal route(26)was chosen in the present study as numerous ocular alterations related to other methods have been reported in previus studies. In corroboration with previous studies(26) in rabbits, the clinical signs associated with uveitis, such as conjunctival hyperemia, chemosis, blepharospasm, and ocular discharge, were also observed in the present study.
Nussenblatt et al. (26) reported a 3-fold increase in flare values in rabbit eyes at 3 hours after LPS injection before decreasing 9 hours later. However, no significant changes in flaremetry were observed following endotoxin intravitreal injection in the present study. As albino rabbits were used, the absence of pigmentation in the uveal tract may have influenced the results of the present study.
Inflammatory mediators have been shown to act as vasoactive substances contributing to conjunctival hyperemia, injection of episcleral vessels, and engorgement of iris vessels(7) during the uveitis evolution process. The inflammatory process occurs as a tissue response to cell injury(28)initiated by the release of pro-inflammatory agents, particularly IL-1 and TNF-α, which stimulate the production of metabolites and cytokines and the liberation of arachidonic acid, PGs, and leukotrienes(27).
In the present study, compared to baseline values, the levels of IL-1 and TNF-α and not of MPO and PGE2 increased after the induction of uveitis; this can be attributed to evaluations being performed during the initial phase of inflammation. The results of the present study corroborate with those of previous studies that have used LPS to induce inflammation(28) and increase levels of TNF-α and IL-1beta. TNF-α, IL-1beta, IL-2, IL-6, and interferon-gamma have also been detected in eyes with idiopathic uveitis. Studies in humans and rats have reported increased expression of TNF-α in tissues adjacent to the infiltration of inflammatory cells(29). In contrast, Pinard et al.(12)observed no significant increases in TNF-α levels in dogs with paracentesis-induced uveitis.
Bonfiglio et al. (30) reported that local instillation of varying concentrations of morphine causes variable intraocular alterations; for example, they observed that 1% morphine instillation, as used in the present research, increased nitric oxide production and affected the anterior chamber. These same authors, as well as Russel-Randall and Dortch-meat(18), observed the presence of opioid receptors, particularly mu opioid receptors, in the anterior chamber of the eye.
Endogenous and exogenous opioids, such as morphine, are known to have immunosuppressive effects (22). However, there is a lack of data regarding the anti-inflammatory effects of morphine infusion in the uveal tract. In the present study, local administration of morphine was not found to ameliorate increased PGE2 expression in response to uveitis.
As a limitation of this study, in order to avoid the use of paracentesis, levels of inflammatory mediators in the aqueous humor were not evaluated, which may have biased the results. However, expression levels of TNF-α, IL-1beta, PG2, and MPO were measured in iris and ciliary body samples. Previously, Green et al.(4) observed transient elevations in iris, ciliary body, and choroidal blood flow using fluorescein angiography in rabbits with endotoxin-induced experimental uveitis.
CONCLUSIONS
In the present study, 1% morphine instillation had no effect on clinical parameters or aqueous humor photon counts, as determined by laser flaremetry in rabbits with endotoxin-induced experimental uveitis. No significant differences in the expression levels of TNF-α, IL-1beta, PGE2, or MPO were between values measured before (baseline) and 20 hours after uveitis induction.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

