INTRODUCTION
Tonometry is fundamental to routine ophthalmological evaluation, and Goldmann applanation tonometry (GAT) is the gold standard method. Several devices, including the Perkins tonometer, Tono-Pen, Pascal tonometer (dynamic contour), and non-contact tonometer, can provide reliable values in adults(1-4). However, in general practice, intraocular pressure (IOP) measurement can be difficult in children due to a lack of cooperation.
Several conditions, such as childhood glaucoma, uveitis, and ocular trauma, can present elevated ocular pressure. When a routine IOP measurement is not possible, the procedure is often conducted under general anesthesia. However, general anesthesia is associated with a number of complications. Studies have revealed increased mortality associated with IOP measurement under general anesthesia in children compared with that in adults; the procedure may also be associated with long-term adverse neurodevelopmental effects(5-7).
The Icare tonometer (Icare, Helsinki, Finland) is a portable tonometer that does not require topical anesthetics and causes minimal discomfort during examination(8). This device is based on the impact and rebound of a probe against the cornea(9). Previous studies have demonstrated a strong agreement between IOP measurements obtained by rebound tonometry (RBT) and GAT. Moreover, the variables that influence reliable measurement are the same for both devices(10,11).
By providing greater comfort during IOP measurement, the Icare tonometer is generally well tolerated by children. Sahin et al. evaluated the levels of discomfort during IOP examination in school children, with 93.4% reporting no discomfort and 6.6% reporting slight discomfort(12). Flemmons et al. reported that it was possible to acquire a reliable IOP measurement on the first attempt in over 93% of the examined children(13).
The purpose of the present study was to evaluate and compare the feasibility, length of the examination, and corneal epithelial damage induced by tonometry with RBT versus GAT in school children. The level of agreement between the two methods and the correlation between IOP and the central corneal thickness (CCT) were analyzed.
METHODS
This protocol was approved by the Ethical Committee of the Federal University of São Paulo and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice(14). Informed parental consent was obtained for all of the enrolled participants before the study.
Healthy children aged between 6 and 8 years were recruited through a general ophthalmological office after refractive error screening. Exclusion criteria included logMAR vision worse than 0.7, a history of ocular disease, medication use, and corneal epithelial defects. All children underwent slit-lamp examination, and those found to have corneal pathology were excluded.
The subjects were randomly selected using a sequentially numbered, opaque sealed-envelope technique(15) to undergo IOP measurement by GAT followed by RBT (study arm 1) or RBT followed by GAT (study arm 2). Tonometric examination with the second device was performed 5 min following the end of the first examination. All of the IOP measurements were conducted by glaucoma specialists with pediatric care experience, and all of the tonometric evaluations and corneal status assessments were performed by three masked examiners in three different offices.
Anesthesia was not required for IOP measurement with RBT. The subjects were instructed to look straight ahead, and the instrument was positioned with the tip of the probe at a distance of 4 mm to 8 mm from the corneal apex. For RBT, when the operator activates the tonometer, six measurements are automatically performed, and the mean value is calculated. After topical administration of anesthesia and fluorescein eye drops, GAT measurement was performed using a slit lamp. The length of time taken for each examination was quantified with a chronometer and included the time taken for administering the drops, explaining the examination procedure, and performing the measurements. The number of attempts with each tonometric method was recorded, with a maximum of three attempts allowed to measure IOP in each eye for each tonometer. Only RBT readings with a high level of reliability were used. After the third attempt, if the subject did not allow the examination to be performed, the test was considered to be unsuccessful. The right eye was measured first for both tonometers in all subjects.
Corneal epithelial damage was assessed after the first tonometric method (RBT or GAT) of IOP measurement using biomicroscopic examination of the type and extent of staining. When RBT was performed first, the examiner instilled anesthetic and fluorescein drops after the measurement to mask the second examiner for the assessment of the corneal status of the eye. The corneal surface was divided into five zones of equal area (central, nasal, superior, inferior, and temporal). The type of staining in each zone was classified as absent, micropunctate, macropunctate, coalescent macropunctate, or patch. The extent of staining in each zone was graded 0 (absent), 1 (1%-15% of the surface), 2 (16%-30% of the surface), 3 (31%-45% of the surface), or 4 (≥46% of the surface).
The CCT was determined using a central ultrasonic pachymeter (Alcon Laboratories Inc., Fort Worth, TX, USA). Under topical anesthesia, the seated patient was asked to look at a distant target ahead. The pachymeter probe was aligned perpendicularly and central to the pupil; the mean of five measurements was calculated.
All statistical analyses were performed using GraphPad Prism version 6.00 for Mac (GraphPad Software, La Jolla, CA, USA). Pooling of data from the right and left eyes during statistical analysis may cause bias as they are not independent variables; therefore, to avoid such bias, only data from the right eyes were analyzed. Moreover, a Bland-Altman analysis was performed to examine the level of agreement between the two devices. The differences between the category frequencies were evaluated by chi-square tests, and paired analysis of nonparametric measures was performed using the Wilcoxon matched-pairs signed-rank test. Student'st-tests were used to analyze data with a normal distribution. Correlations between variables were evaluated by Pearson's correlation coefficients. The level of statistical significance was set at p<0.05.
RESULTS
In the present study, 36 (63.2%) out of 57 patients were male and 21 (36.8%) were female. The mean age of the children was 6.76 ± 0.38 years, and the mean uncorrected visual acuity was 0.85 ± 0.11 logMAR.
To achieve a reliable measurement, more attempts were made with GAT than RBT (1.54 ± 0.69 vs. 1.13 ± 0.34, p=0.0021) in both study arms. All children permitted IOP measurements with at least one tonometer. More children did not allow the examination to be performed with GAT than with RBT in both study arms (study arm 1: 26%, n=7 vs. 4%, n=1, p<0.001; study arm 2: 16%, n=5 vs. 6%, n=2, p<0.001; chi-square test). Examination with RBT was faster than that with GAT (67.81 s ± 35.20 s vs. 126.70 s ± 56.60 s,p<0.0001).
IOP measurements quantified with RBT were statistically different from those quantified with GAT in both study arms 1 (15.20 ± 2.74 mmHg vs. 13.25 ± 2.47 mmHg,p=0.0247) and 2 (16.76 ± 3.98 mmHg vs. 13.92 ± 2.08 mmHg;p=0.0003; Table 1).Figure 1 shows the Bland-Altman plots of IOP and the linear regression of these values. A positive correlation between the difference and the mean IOP measurements was observed in study arm 2 (r=0.6347, p=0.0007; Figure 1 B). No statistically significant difference was observed in the degrees of bias in study arms 1 and 2 (1.95 ± 3.58 mmHg vs. 2.84 ± 3.37 mmHg, respectively).
Table 1 Intraocular pressure as measured by GAT and RBT
| Method | Mean* | SD | Minimum | Median | Maximum |
|---|---|---|---|---|---|
| Study arm 1 (n=20) | |||||
| GAT | 13.25 | 2.47 | 10 | 14 | 19 |
| RBT | 15.20 | 2.74 | 11 | 15 | 21 |
| Study arm 2 (n=25) | |||||
| GAT | 13.92 | 2.08 | 10 | 14 | 17 |
| RBT | 16.76 | 3.98 | 9 | 18 | 24 |
RBT= rebound tonometry; GAT= Goldmann applanation tonometry; SD= standard deviation.
*All recorded values of intraocular pressure are presented in mmHg. The difference between GAT and RBT was statistically significant (p<0.05; paired t-test) in both study arms.
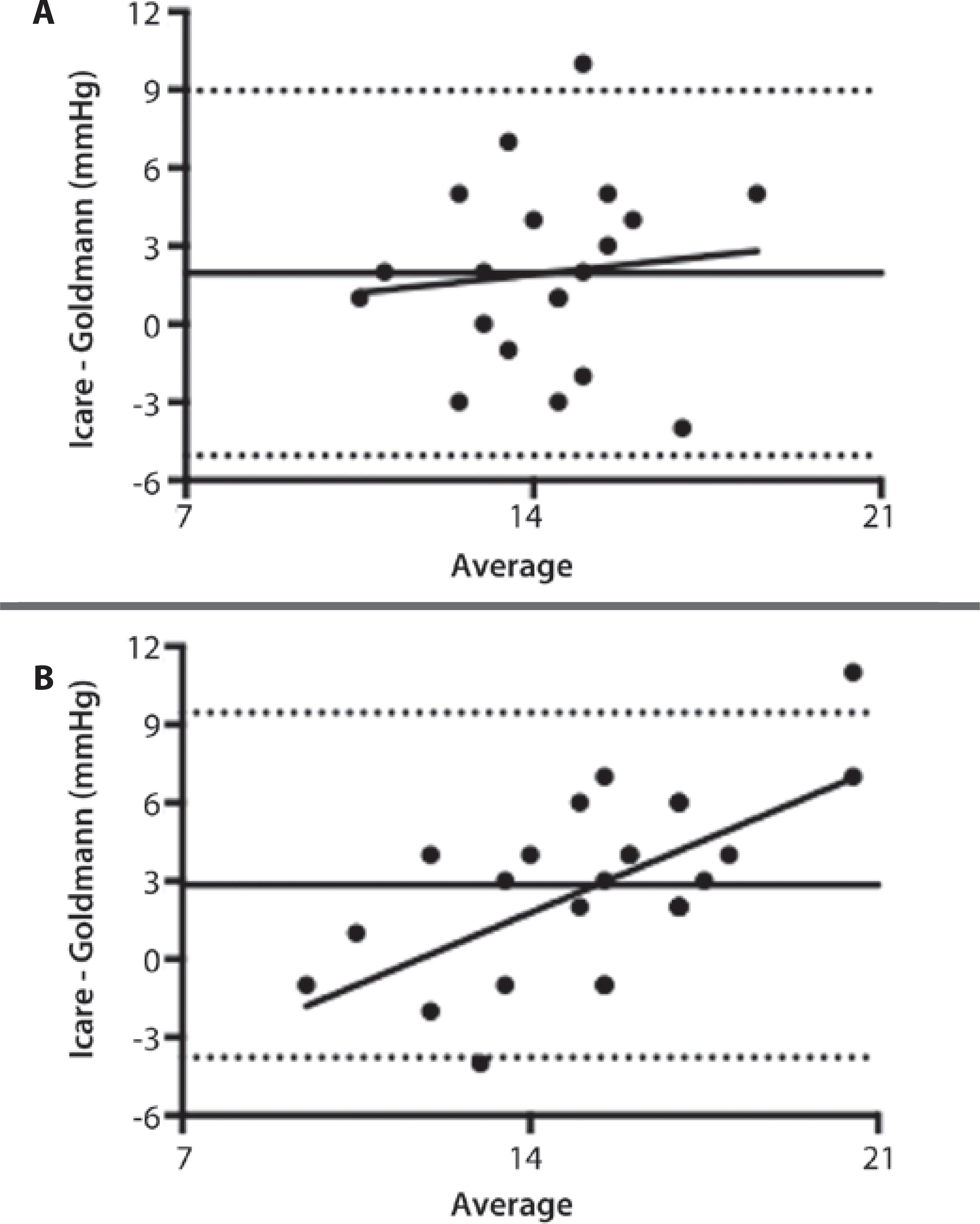
Figure 1 Bland-Altman analyses of IOP measurements between rebound tonometry (RBT) and Goldmann applanation tonometry (GAT) in study arms 1 (A) and 2 (B). The mean bias (solid line) and 95% limits of agreement (dashed lines) are shown. In study arm 1 (A), the mean bias was 1.95 ± 3.58, and the correlation between the differences and the mean values was not significant (r=0.1062, p=0.6558). In study arm 2 (B), the mean bias was 2.84 ± 3.37, and a positive correlation was observed between the differences and the mean values (r=0.6347,p=0.0007).
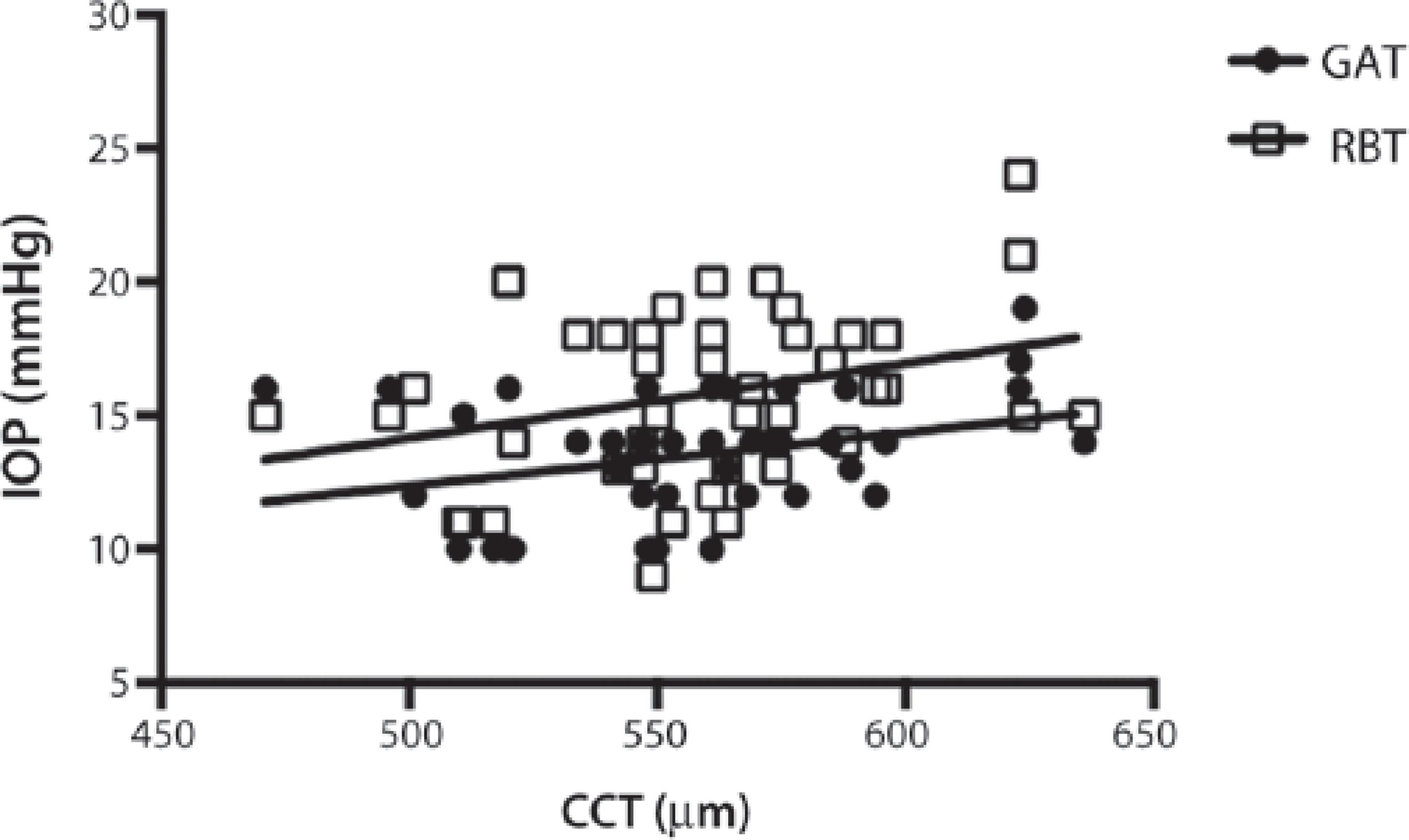
The mean CCT was 558 ± 36 µm, and a positive correlation was found between CCT and IOP for GAT (r=0.3163, p=0.0388) and RBT (r=0.3080, p=0.0445; Figure 2).
The frequency of corneal epithelial damage induced by RBT and GAT was similar (13% vs. 11%; chi-square test), and the most common type of staining was micropunctate on less than 16% of the corneal surface (grade 1). Only one patient who underwent RBT examination presented staining of 16% to 30% of the surface (grade 2), and this was located in the inferior corneal zone.
DISCUSSION
In clinical practice, IOP measurement is often impaired by a lack of cooperation by school children. The present study revealed good tolerance for RBT, with only <6% of the examined children refusing the tonometry. Moreover, the examination was less time consuming, and the number of attempts required was fewer than that with GAT. RBT was better tolerated as it can be performed without administering anesthesia. Due to the reduction of discomfort, tonometric examination could be successfully performed in more children. The impact of the tonometer tip is extremely gentle in RBT, and frequently does not provoke an eye-blinking reflex(9).
Although IOP measurements with RBT were statistically significantly higher than those with GAT, this difference was not clinically relevant. Several authors have reported a good level of agreement between the two tonometers, with RBT generally overestimating IOP by around 3 mmHg(16-18). The difference between the acquired values by the two tested tonometers was higher for high mean IOP values than that for low mean IOP values. Furthermore, in accordance with previous studies, we observed positive correlations between IOP measured by both devices and CCT(19-21).
Despite a small number of children being uncooperative during IOP measurements, neither of the tonometers produced marked corneal epithelial defects. This may be because ophthalmologists that conducted the examinations were experienced in pediatric care.
The small number of children enrolled limited our study. As only healthy children were evaluated, these results may not be applicable to patients with childhood glaucoma or other ophthalmic diseases. Further, the corneal applanation induced by GAT may have influenced IOP measurements when RBT was performed second (study arm 1). However, only the influence of corneal thickness was evaluated in the present study.
CONCLUSION
IOP was successfully measured in all children with at least one of the tonometers. RBT was better tolerated and was faster than GAT examination. RBT did not induce epithelial lesions, although it overestimated IOP by around 3 mmHg. However, we do not believe this to be a clinically relevant disadvantage of RBT. In routine clinical settings, GAT remains the gold standard for the measurement of IOP; however, RBT may be a useful screening tool for non-cooperative patients, such as school children, and may allow avoidance of the use of general anesthesia for IOP measurement. High RBT measurements should be corroborated by assessment of the clinical presentation, and IOP should be measured by other tonometric methods.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

