INTRODUCTION
Keratoconjunctivitis sicca (KCS), or dry eye, is characterized by decreased tear quantity and/or quality. Clinical signs of KCS include ocular discomfort, dryness, irritation, foreign body sensation, light sensitivity, itching, and in severe cases, corneal ulceration, which can lead to blindness. Immune-mediated inflammation of the lacrimal glands is one of the most frequent causes of dry eye. The use of topical immunosuppressive drugs such as cyclosporine, tacrolimus, and pimecrolimus, alone or combined with artificial tears, are indicated; other agents, including corticosteroids, essential fatty acids, mucolytics, pilocarpine, and antibiotics, may be used as adjuvants or if the patient fails to respond to primary lacrimogenic therapy(1).
Cyclosporine A (CsA), derived from the asexual ascomycete fungus Tolypocladium inflatum, is a noncytotoxic immunosuppressor that inhibits the proliferation of T lymphocytes by inhibiting interleukin-2 synthesis at the transcription level(2,3). CsA use increases tear secretion and the number of goblet cells and decreases the incidence of corneal staining by interrupting the autoimmune anti-inflammatory reaction(1,4). CsA eye drops are marketed in concentrations up to 0.05% in polivinilic alcohol, of 0.05% in an anionic ophthalmic emulsion (Restasis®; Allergan, Irvine, CA, USA; glycerin base, castor oil, and polysorbate 80), of 1% and 2% in eye drops in an oily vehicle (olive oil), and of 0.2% in a veterinary ophthalmic ointment (Optimmune®; Schering Plough, Summit, NJ, USA)(2,5-7). Some adverse effects of ocular topical CsA in humans include allergy, burning sensation, and irritation of the conjunctiva(6,7).
Olive oil, from the fruit of the olive tree Olea europaea, contains a significant concentration of monounsaturated fatty acids (MUFAs), of which 70-80% are oleic acid, in addition to 10-15% saturated fatty acids (palmitic acid) and 5-10% omega 3 (ω-3), 6 (ω-6), and 9 (ω-9) polyunsaturated fatty acids (PUFAs)(8). Olive oil has been shown to possess antinociceptive, anti-inflammatory, antiarrhythmic, spasmolytic, immunostimulant, cardioprotective, hypotensive, hypoglycemic, and antimicrobial properties(9-11).
Linseed oil, from the flax plant Linum usitatissimum, is composed of 57% ω-3, 16% ω-6, 28% MUFA, and only 9% unsaturated fatty acids; the ω-3-to-ω-6 ratio of 1:3 is considered to be close to ideal(12). Linseed oil is given orally as an adjuvant therapy in patients with Sjögren's syndrome and KCS(13,14). Some authors have reported the treatment of experimentally induced KCS in rabbits using linseed oil in various preparations (oral, topical, and oral/topical combination)(15,16). In mice with experimentally induced KCS, the topical use of ω-3 and ω-6 has been shown to successfully control inflammatory symptoms(17). Linseed oil is considered a natural anti-inflammatory agent due to its potential for synthesizing noninflammatory mediators, such as prostaglandin E1 (PGE1) and thromboxane A1 (TXA1)(18,19).
The objective of this study was to evaluate the effectiveness of cyclosporine, a potent immunosuppressor used in the treatment of KCS, as 1% eye drops diluted in olive oil (already used as a vehicle for cyclosporine) or linseed oil, in addition to the use of each oil separately (without KCS), in the treatment of experimentally induced KCS in rabbits.
METHODS
Animals
This experiment was approved by the Ethics Committee on the Use of Animals of UNOESTE (protocol nº 720/11). Twenty-five male New Zealand white rabbits (Oryctolagus cuniculus) of a mean age of 198 days and a mean weight of 3,600 g were selected from the Central Bioterium of UNOESTE and kept in individual metal cages with access to water and food ad libitum.
The induction of KCS
The induction model of KCS in rabbits was based on previously published studies that used 1% atropine sulfate eye drops three times daily(15,16,20-22). Atropine is a competitive antagonist of the muscarinic acetylcholine receptors. As the ocular surface and main lacrimal gland are innervated by parasympathetic fibers of the seventh cranial nerve; the use of atropine is known to cause decreased tear secretion and consequent inflammation in a similar way to dry eye syndrome(20-22). Eye drops were administered until the diagnosis of KCS (7 days to induce KCS in all rabbits) was confirmed by a Schirmer tear test 1 (STT; Teste de Schirmer® Ophthalmos Laboratory) result of ≤5 mm/min and/or ≤10 seconds tear-film break-up time (TBUT)(23,24) and throughout the treatment period (12 weeks) for KCS maintenance.
Groups
The data from both eyes were pooled from the 25 rabbits. Of these rabbits, 20 were induced using the KCS protocol, and 5 were allocated to the non-induced control group. The timing of the 1% atropine eye drop instillation was 6:00 am, 2:00 pm, and 10:00 pm, and the timing of the treatment eye drop instillation was 8:00 am and 8:00 pm. Beginning 1 week after KCS induction, the animals were treated for 12 weeks as follows: Group C (control; n=5) (one drop of placebo, 0.9% NaCl solution [Fresenius Kabi, Campinas, São Paulo, Brazil], topically applied twice a day [BID] in both eyes); Group CO (one drop of 1% cyclosporine in olive oil [Ophthalmos®, São Paulo, Brazil], topically applied BID in both eyes); Group CL (one drop of 1% cyclosporine in linseed oil [Ophthalmos®], topically applied BID in both eyes); Group O (one drop of olive oil [Ophthalmos Laboratory], topically applied BID in both eyes); and Group L (one drop of linseed oil [Ophthalmos Laboratory], topically applied BID in both eyes). The rationale behind using 1% CsA was that it is a manipulated concentration marketed by Ophthalmos Laboratory in Brazil.
Ocular tests
The following ocular tests were performed at time point T0 (before the induction of KCS), and at weeks 1 (T1), 2 (T2), 4 (T4), 8 (T8), and 12 (T12) post-induction, always in the morning (10:00 am). One blinded observer evaluated the ocular tests. All tests were performed concurrently.
STT was performed without anesthetic eye drops, and was used to determine the quantitative tear production.
TBUT for evaluation of the quality of the tears was performed twice, successively, and the mean was calculated. After placing a drop of 1% fluorescein eye drops (Fluoresceína®; Allergan, São Paulo, Brazil) into the lower fornix, a slit-lamp, set on a bright light setting with a cobalt blue filter, was used to measure the time between the last blink and the first appearance of a dark spot on the cornea (formation of a dry area).
The fluorescein test (FT) was performed with 1% fluorescein strips (Fluoresceína strips®; Ophthalmos Laboratory) moistened with a small amount of normal saline to observe stained corneal epithelial irregularity in four quadrants (temporal superior, temporal inferior, nasal superior, and nasal inferior) and was scored 0 to 4: (0) no staining; (1) one quadrant stained; (2) two quadrants stained; (3) three quadrants stained; and (4) four quadrants stained.
The rose bengal test (RBT) was performed to stain tissue devoid of mucus. Anesthetic eye drops (0.5% proxymetacaine, Anestalcon®; Allergan, São Paulo, Brazil) were instilled followed by one drop of 0.5% rose bengal (Colírio de Rosa Bengala®; Ophthalmos®). The following scoring scale was used from 0 to 3: (0) no staining; (1) only conjunctival staining; (2) only corneal staining; and (3) both conjunctival and corneal staining.
Histopathological analysis
For the histopathological analysis, the rabbits were euthanized at the end of the experiment (T12) using 2.5% sodium thiopental (200 mg/kg) administered intravenously. After transpalpebral enucleation, the eyeball was placed in a 10% formaldehyde solution for 24-48 hours. The eyes were stored in 70% alcohol, and then routinely processed and embedded in paraffin. Three serial sections of 5 µm from the cornea and conjunctiva were obtained and stained with hematoxylin and eosin (HE) and periodic acid-Schiff (PAS). The cornea and conjunctiva were assessed for the following: edema, cellular degeneration, necrosis, inflammatory infiltrate (neutrophilic, mononuclear, and mixed), and goblet cell count (bulbar conjunctiva). Edema, cellular degeneration, and necrosis were scored from 0 to 3: (0) absence, (1) mild, (2) moderate, and (3) severe. Inflammatory infiltrate was scored from 0 to 3: (0) absent, (1) mild (1-4 cells per field), (2) moderate (5-9 cells per field), and (3) severe (>10 cells per field). Goblet cell density (cells/mm2) was counted in five high-power fields (40× objective) on each slide, corresponding to an area of around 0.5 mm2. One blinded observer evaluated the histopathological exams.
Statistical analysis
The STT and TBUT values and goblet cell density were compared between the groups and time points using analysis of variance (ANOVA), in addition to Tukey's method. FT and RBT values, were analyzed with the Friedman and Kruskal-Wallis tests, for comparisons within groups over time and among different groups, respectively. A statistical significance level of p<0.05 was adopted. The software used for the statistical analysis was Biostat 5.3.
RESULTS
Group C exhibited normal parameters throughout the entire experimental period. In the treatment groups, STT values at T1 were lower than those at T0 for group O (3.7 ± 1.3; p<0.01), group L (4.1 ± 1.6; p=0.01), group CO (3.6 ± 2.1; p<0.01), and group CL (4.2 ± 1.3; p=0.01). STT values increased in all treatment groups at T2, but were still lower than T0 in groups O (5.4 ± 1.0; p<0.01) and group CL (6.4 ± 2.4; p<0.01). There was no statistically significant difference at T4, T6, T8 and T12 in any of the groups in relation to T0 (p>0.05) (Figure 1 A). TBUT values decreased at T1 for group O (3.8 ± 1.4; p<0.01), group L (4.4 ± 1.7; p<0.01), group CO (4.9 ± 0.7; p=0.01), and group CL (4.8 ± 1.7; p=0.01). TBUT values increased at T2 in all treatment groups but were still lower than T0 in groups O (13.3 ± 3.9; p<0.01), CO (12.6 ± 3.3; p<0.01), and CL (13.5 ± 3.3; p<0.01). There was no statistically significant difference at T4, T6, T8 and T12 in all groups when compared to T0 (p>0.05) (Figure 1 B).
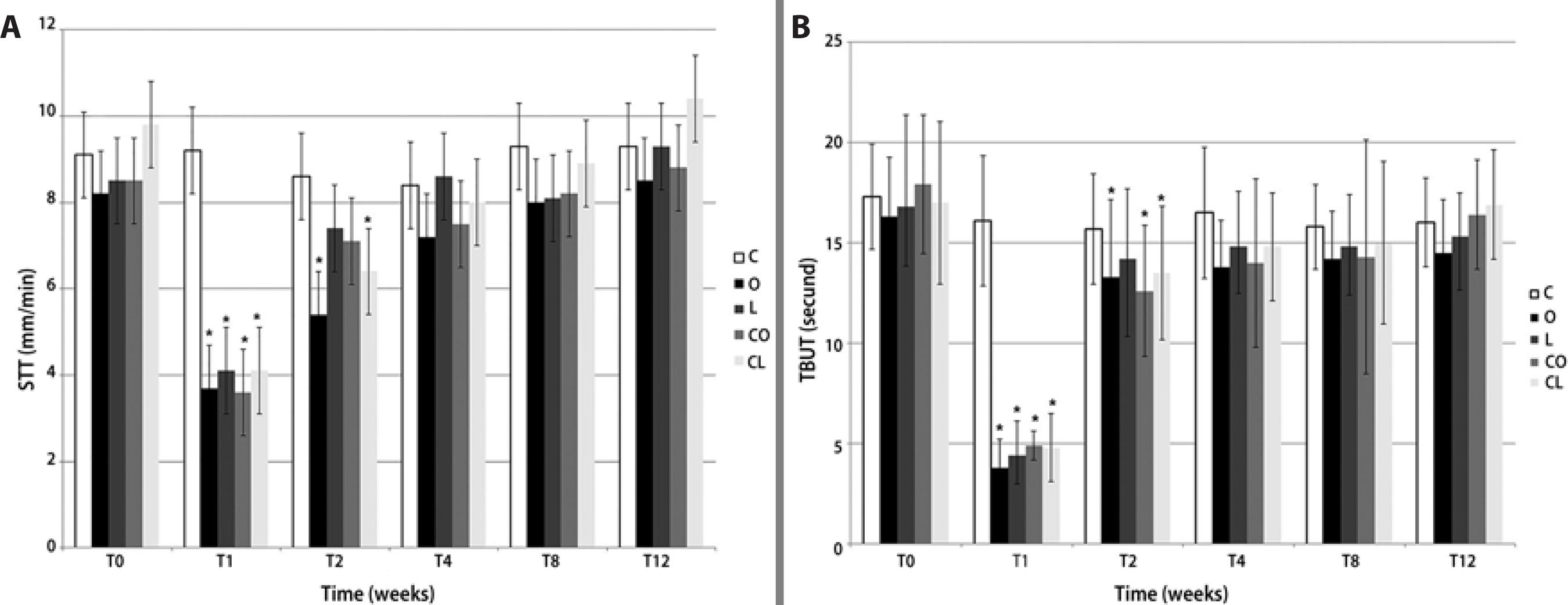
*P<0.05 (Tukey's test, compared to control group).
Figure 1 Mean ± SD for (A) Schirmer's tear test I (STT I)a in mm/min and (B) tear-film break-up time (TBUT)b in seconds, in rabbits with experimentally induced keratoconjunctivitis sicca (KCS) that were treated with various topical formulations: placebo (group C), olive oil (group O), linseed oil (group L), 1% cyclosporine in olive oil (group CO), and 1% cyclosporine in linseed oil (group CL).
The FT results are presented in figure 2 A. At T1, only group O showed an FT score of 2 (two stained quadrants), while the other groups: L, CO, and CL, showed an FT score of 1 (one stained quadrant). At T2 and T4, all treatment groups presented an FT score of 1, and at T8 and T12, no eyes showed positive staining. The RBT results are shown in figure 2 B. From T1 to T4, all treatment groups presented a score of 2 (only corneal staining). At T8, groups O, L, and CO presented scores of 2, and at T12, no eyes were RBT positive.
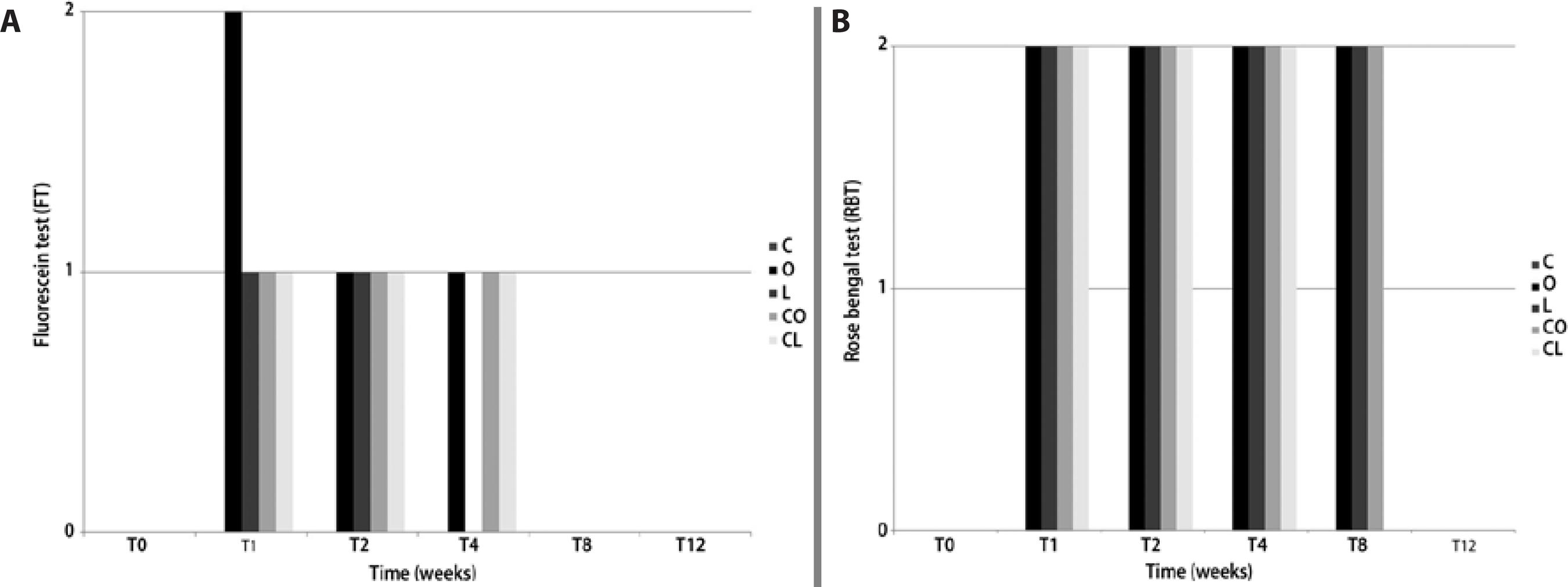
a= (0) no staining, (1) one quadrant stained, (2) two quadrants stained.
b= (0) no staining, (1) only conjunctival staining, (2) only corneal staining, (3) conjunctival and corneal staining.
Figure 2 Median results for (A) fluorescein test (FT)a and (B) rose bengal test (RBT)b in rabbits with experimentally induced keratoconjunctivitis sicca (KCS) that were treated with various topical formulations: placebo (group C), olive oil (group O), linseed oil (group L), 1% cyclosporine in olive oil (group CO), and 1% cyclosporine in linseed oil (group CL).
The histopathological images of the cornea and conjunctiva are presented in figure 3. A normal cornea from group C is shown in figure 3 A. Slight edema and conjunctival congestion were found in groups O (Figure 3 B) and CO. Regarding the goblet cell density (Figures 3 Cand D), there was no statistically significant difference between the groups (p=0.147), although group CL appeared to have higher values (11.2 ± 3.5) compared to groups C (8.0 ± 2.1), O (7.6 ± 3.7), L (9.0 ± 3.4), and CO (8.4 ± 3.2). The histopathological results of all the conjunctival samples from group CO (Figure 3 E) and O (Figure 3 F) showed more chronic inflammation than the other treatment groups.
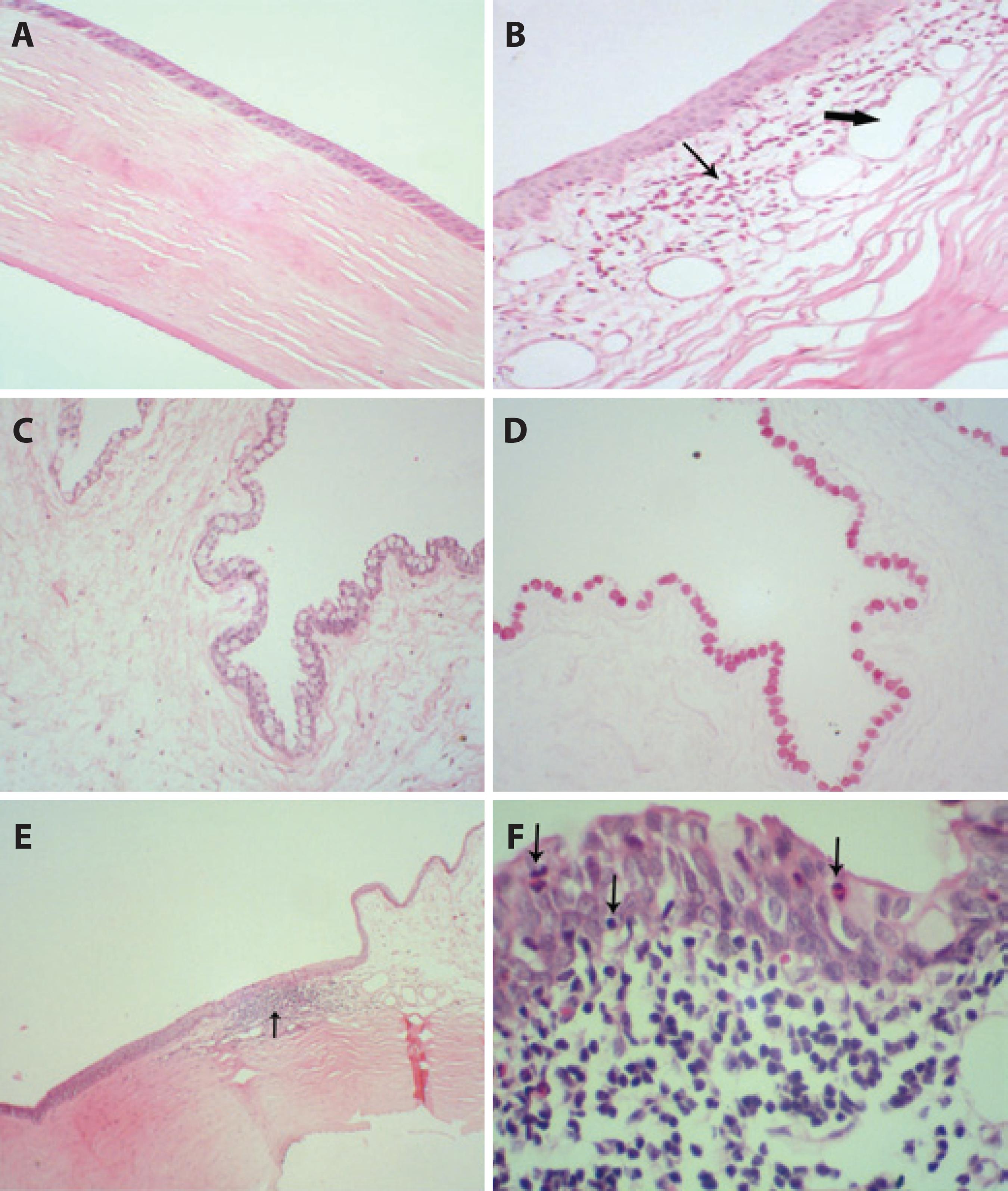
Figure 3 Photomicrographs of the cornea and conjunctiva at T12. (A) Normal cornea from group C (control) (hematoxylin and eosin [H&E]; 100×). (B) Conjunctiva with eosinophil infiltration (thin arrow), edema, and vascular congestion (capillary dilatation-large arrows) in group O (olive oil) (H&E; 100×). (C) Conjunctiva with a large number of goblet cells in group CL (1% cyclosporine in linseed oil) (H&E; 100×). (D) Goblet cells from group CL (1% cyclosporine in linseed oil) (periodic acid-Schiff [PAS]; 100×). (E) Focal chronic inflammatory process in the transition from the conjunctiva to the cornea (arrow) in group CO (1% cyclosporine in olive oil) (H&E; 40×). (F) Details of the chronic inflammation in the conjunctiva with aggression of the epithelium (presence of inflammatory cells within the epithelium) (arrows) in group O (olive oil) (H&E; 400×).
DISCUSSION
When comparing the two different oils, linseed oil showed a more favorable and rapid effect than olive oil in terms of the STT and TBUT results at T2, FT results at T4, and in the histopathological results. In general, Group O showed the worst results in the treatment of KCS. These variations could be explained by the difference in composition for each oil. Olive oil contains a larger quantity of MUFA (70-80% oleic acid) and only a small amount of PUFA (5-10% of ω-3, ω-6, and ω-9)(10,11). Linseed oil is comprised of 16% ω-6 and 57% ω-3, which are natural anti-inflammatory agents due to their ability to synthesize noninflammatory mediators(12,25). The ω-6 origin of PGE1 and TXA1 via cyclooxygenase 1 (COX1) and the effects of the ω-3 fatty acids eicosapentaenoic acid and docosapentaenoic acid could explain the clinical improvements in the animals that received the linseed oil treatment(18,19).
These results are in agreement with a previous study that used a topical formulation containing an emulsion of alpha-linolenic acid and linoleic acid on mice with experimentally induced KCS; the inflammatory symptoms of KCS improved in the mice(17). A recent study compared almond and linseed oil as diluents of tacrolimus, another immunosuppressor, in the treatment of KCS in rabbits and showed that linseed oil either as a diluent or in isolation was more effective than almond oil(16). Another study evaluated the effectiveness of various linseed oil preparations (oral, topical, and an oral/topical combination) in treating experimentally induced KCS in rabbits, and concluded that orally or topically administered linseed oil was effective in the treatment of KCS, although the combined oral/topical treatment did not show additional benefits(15).
When the CsA groups were compared with the oil groups, groups CL and CO were found to present similar TBUT and FT results at the end of the experiment. Additionally, there was more rapid negative RBT staining for group CL, which also had higher, although non-statistically significant, goblet cell density. The better results in group CL for these parameters might be due to the combination of the anti-inflammatory properties of the linseed oil and the immunosuppressive effects of the cyclosporine. This result is in agreement with the findings from the study that used tacrolimus, another immunosuppressor used in KCS treatment; the group that was administered tacrolimus diluted with linseed oil showed better improvement in KCS control in rabbits, with increased goblet cell density in the conjunctiva(16). In rabbits with KCS induced by autoimmune dacryoadenitis, the use of 0.05% CsA in an ophthalmic emulsion (Restasis®) promoted significantly increased tear production(26). These CsA effects are due to the immunosuppressive action that minimizes the inflammatory damage induced by KCS in the cornea, conjunctiva, and goblet cells(2-5). The increased density of goblet cells in KCS is very important because decreased goblet cell density can induce mucin deficiency in the precorneal tear film and may result in ulcerated corneas with irregular surfaces(4,27).
The histopathological results also demonstrate a stronger inflammatory process in the O and CO groups (Figures 4 E and F), as assessed by the presence of mild edema in the cornea and moderate edema and congestion in the conjunctiva. Additionally, only group O demonstrated squamous metaplasia, which was most likely due to chronic ocular inflammation(1,2,5). The goblet cell density was not significantly increased in group CL when compared to the other groups, but this difference could potentially become statistically significant in a larger population.
We conclude that cyclosporine diluted in olive oil and linseed oil is effective for the treatment of experimentally induced KCS in rabbits. However, linseed oil, when used alone or as a diluent for cyclosporine, is more effective at treating KCS than olive oil. This study reveals that both the immunosuppressive effect of cyclosporine and the properties of the oil medium, particularly the anti-inflammatory properties of linseed oil, are responsible for the improvement of dry eye symptoms in rabbits. These results may contribute to the development of novel topical ophthalmic formulations for the treatment of KCS in future.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

