INTRODUCTION
High myopia is a common condition worldwide, defined as an axial length of ≥26.0 mm. The frequency of vitreous and retinal degenerations, such as liquefied vitreous, posterior vitreous detachment, lattice degeneration, retinal holes, and retinal tears, is higher in patients with high myopia than in the general population(1). The World Health Organization estimated there were 161 million visually impaired people worldwide in 2002, with cataract accounting for 47.8% of cases and the incidence of cataract increasing with age. In developing countries, cataracts occur earlier in life and the incidence is higher(2). Highly myopic eyes tend to develop cataracts earlier than normal eyes, with higher prevalences of nuclear and posterior subcapsular cataracts. In a retrospective study, the prevalence of nuclear and posterior subcapsular cataracts were reportedly 25.07% and 11.82%, respectively, in non-myopic patients and 40.63% and 26.22%, respectively, in highly myopic patients(3-5). The selection of appropriately powered intraocular lens (IOL) implants is critical in maintaining good visual quality(6).
Both cataract surgery and high myopia increase the risk of postoperative rhegmatogenous retinal detachment (RRD), which originates from retinal holes, tears, or breaks. The degree of myopia, axial length, intraoperative posterior capsule rupture, vitreous loss, and postoperative Nd:YAG laser capsulotomy applications have all been shown to increase the risk of RRD(7).
In this study, we evaluated the outcomes and complications following phacoemulsification surgery in eyes with cataracts and high myopia.
METHODS
The study protocol was approved by the local ethics committee. Informed consent was obtained from all study participants. This study was performed in accordance with the tenets of the Declaration of Helsinki.
In this study, we retrospectively evaluated the data of 43 eyes of 28 consecutive patients with cataract and high myopia (12 males, 16 females) who underwent operative interventions between February 2008 and December 2011. Patients with any systemic or ocular disease other than high myopia that may influence visual acuity, intraoperative complications, or retinal detachment were excluded.
Preoperative and postoperative uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) were measured. Preoperative and postoperative refractive values were measured as spherical equivalent (SE), calculated as the summation of the spherical refractive value and half of the cylindrical value. Axial length measurements were performed using an IOL Master Optical Biometer (Carl Zeiss Meditec AG, Jena, Germany). The SRK/T formula was used for IOL power calculations. Fundus examinations were performed preoperatively. Prophylactic argon laser photocoagulation was performed in 3 eyes diagnosed with retinal tears, 2 eyes with retinal holes, and 2 eyes with lattice degeneration.
Target postoperative refractions were within ±1.00 diopters (D). Twenty-one patients (75%) requested mild myopia postoperatively to maintain a near-sighted lifestyle. However, we predominantly aimed for postoperative emmetropia as myopia may continue to increase in later life.
Each operation was performed by a single surgeon (SC). Under subtenon anesthesia, a 2.8-mm clear corneal incision was made superotemporally with a steel blade. The anterior chamber was then filled with a dispersive (Hydroxypropylmethylcellulose, Easy Visc, Germany) viscoelastic material. After continuous curvilinear capsulorhexis, hydrodissection and hydrodelineation were performed. Then, a sideport entrance was created with a 19-gauge microvitreoretinal (MVR) knife. The lens nucleus was removed using the “stop and chop” technique (Sovereign Compact, Phacoemulsification System, AMO, USA). Subsequently, the cortex was aspirated with coaxial irrigation/aspiration. The capsular bag was filled with a cohesive (Na Hyaluranate 1.6, Easyluron, Germany) viscoelastic material before a foldable monofocal posterior chamber IOL (Acriva, VSY, Turkey) was implanted in the capsular bag through an injector system. The viscoelastic material was then aspirated completely. The entrances were closed with stromal hydration, and finally, intracameral moxifloxacin was administered for postoperative endophthalmitis prophylaxis. Topical antibiotics 4 times a day, and topical steroids 6 times a day were administered for 1 week postoperatively. Topical steroid doses were then tapered over the subsequent 3 weeks.
Data are presented as mean ± standard deviation. The statistical significance of differences in categorical data and continuous parameters were tested using the chi-square test, paired t-test, and Kruskal-Wallis test. All statistical analyses were performed using commercially available statistical software (SPSS version 22, SPSS, Inc., Chicago, IL). Statistical significance was defined as P<0.05.
RESULTS
Results are presented as [mean ± standard deviation (range)]. Out of the 28 patients included in this study, 12 (42.8%) were male and 16 (57.2%) were female. Fifteen (53.6%) patients had bilateral cataracts and 13 (46.4%) had unilateral cataracts. The age of the patients was 59.20 ± 11.08 (39-77) years. Twenty-five eyes (58.2%) had nuclear cataracts, 9 eyes (20.9%) had cortical cataracts, and 9 eyes (20.9%) had posterior subcapsular cataracts. The frequency of nuclear cataracts was significantly higher than that of other cataract types (P=0.003).
Axial length was 28.97 ± 1.99 (26-33) mm and IOL power was 5.09 ± 4.78 (-3.0 to +14.0) D.
Preoperative SE [-16.48 ± 5.23 (-8.00 to -25.00) D] was significantly higher than the postoperative SE [-1.46 ± 0.93 (0.00 to -3.00) D; P=0.00]. Preoperative BCVA [0.91 ± 0.37 (0.30-1.50) logMAR] was significantly lower than the postoperative BCVA [0.29 ± 0.25 (0.00-1.00) logMAR; P=0.00]. Preoperative and postoperative findings are summarized in table 1.
Table 1 Preoperative and postoperative findings
| Characteristics | Number, percentage, mean (± SD) | Range |
|---|---|---|
| Sex | ||
| Male | 12 (42.8%) | |
| Female | 16 (57.2%) | |
| Age (years) | 59.20 ± 11.08 | 39 to 77 |
| Laterality | ||
| Unilateral | 13 (46.4%) | |
| Bilateral | 15 (53.6%) | |
| Type of cataract | ||
| Nuclear | 25 (58.2%) | |
| Cortical | 9 (20.9%) | |
| Subcapsular | 9 (20.9%) | |
| Axial length (mm) | 28.97 ± 1.99 | 26 to 33 |
| IOL power (D) | 5.09 ± 4.78 | -3.00 to +14.00 |
| Pre spherical equivalent (D) | -16.48 ± 5.23 | -8.00 to -25.00 |
| Post spherical equivalent (D) | -1.46 ± 0.93 | 0.00 to -3.00 |
| Pre BCVA (logMAR) | 0.91 ± 0.37 | 0.30 to 1.50 |
| Post BCVA (logMAR) | 0.29 ± 0.25 | 0.00 to 1.00 |
SD= standard deviation;
IOL= intraocular lens;
BCVA= best-corrected visual acuity;
Pre= preoperative;
Post= postoperative.
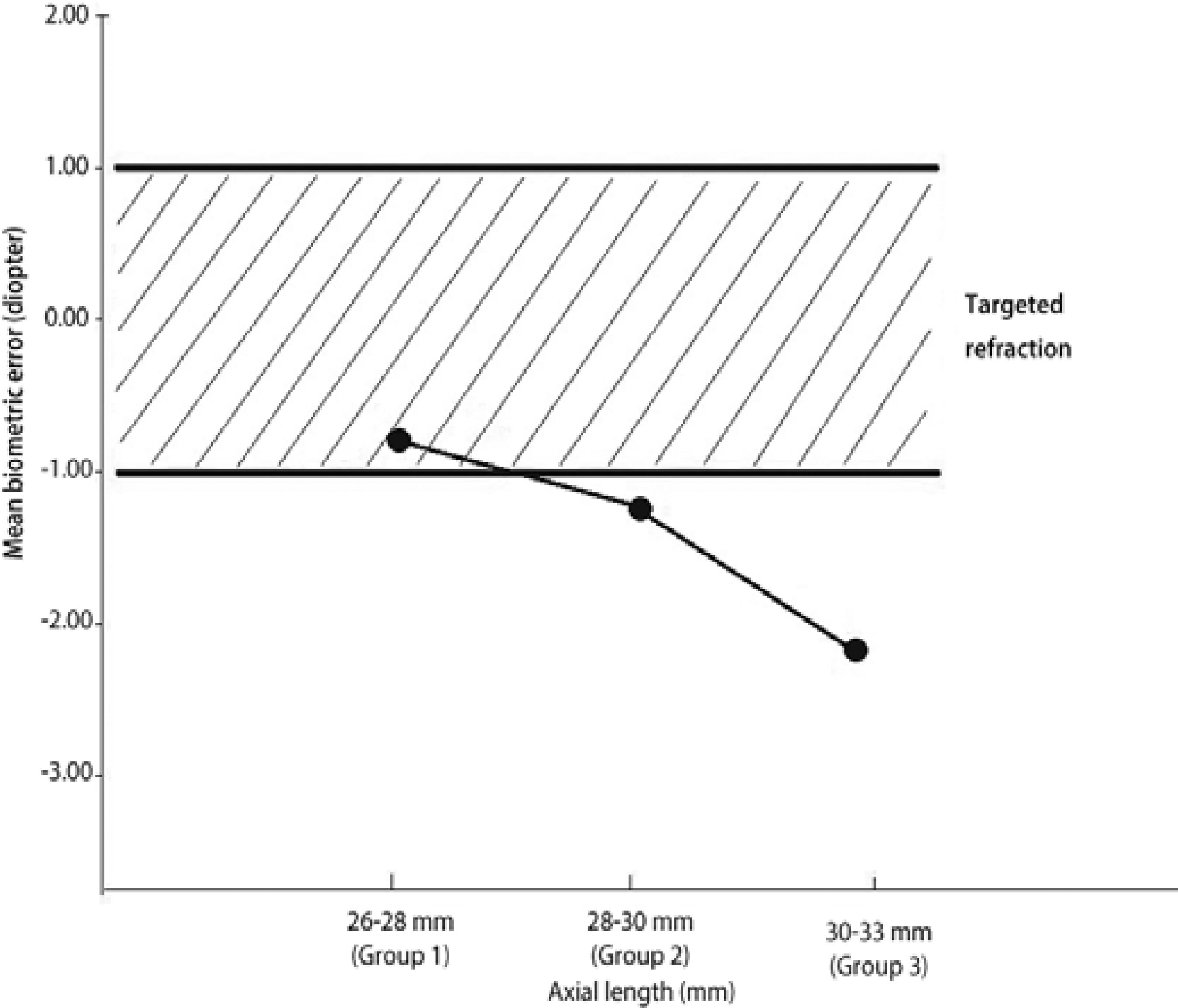
Postoperative follow-up period was 24.44 ± 1.93 (21-28) months. Twenty-two eyes (51.2%) achieved the target postoperative refraction (±1.0 D). Thirty-seven eyes (86%) had refraction within the range of 0.00 to -2.00 D. The eyes were divided into 3 groups according to the axial length. The first group comprised 12 eyes with axial lengths between 26 and 28 mm, the second group comprised 14 eyes with axial lengths between 28 and 30 mm, and the third group comprised 17 eyes with axial lengths between 30 and 33 mm. The mean expected refraction was -0.75 ± 0.33 (0.00 to -1.00) D in Group 1, -0.28 ± 0.32 (0.00 to -1.00) D in \ Group 2, and +0.85 ± 0.23 (+0.50 to +1.00) D in Group 3. The mean obtained postoperative refraction was -1.66 ± 0.88 (0.00 to -3.00) D in Group 1, -1.35 ± 1.00 (0.00 to -3.00) D in Group 2, and -1.41 ± 0.93 (0.00 to -3.00) D in Group 3 (Table 2). The mean biometric error was -0.91 ± 0.84 (0.00 to -2.50) D in Group 1, -1.21 ± 0.77 (0.00 to -2.50) D in Group 2, and -2.14 ± 1.27 (+0.50 to -4.00) D in Group 3 (Figure 1). The mean biometric error was significantly higher in the third group than in the other groups (P=0.007).
Table 2 Mean expected and achieved ocular postoperative refraction
| Groups | Preoperative axial lenght range | Expected postoperative spherical equivalent (Mean ± SD) | Achieved postoperative spherical equivalent (Mean ± SD) |
|---|---|---|---|
| Group 1 | 26 to 28 mmHg | -0.75 ± 0.33 D | -1.66 ± 0.88 D |
| Group 2 | 28 to 30 mmHg | -0.28 ± 0.32 D | -1.35 ± 1.00 D |
| Group 3 | 30 to 33 mmHg | +0.85 ± 0.23 D | -1.41 ± 0.93 D |
SD= standard deviation.
Preoperative prophylactic argon laser photocoagulation was performed in 7 eyes (16%) on account of retinal tears, retinal holes, and lattice degeneration. No postoperative retinal complications were observed following preoperative prophylactic argon laser photocoagulation. Retinal tears developed in 2 eyes (4%) 2 and 4 months postoperatively and were treated with argon laser photocoagulation. One eye (2%) developed retinal detachment (RD) 3 months postoperatively and the patient was referred to another center for retinal surgery. Nd:YAG laser capsulotomy was performed in 11 (25%) eyes that developed posterior capsular opacity between 6 and 18 months postoperatively. No further postoperative complications were observed following argon laser photocoagulation or Nd:YAG laser capsulotomy during further follow-up periods. We were unable to obtain satisfactory postoperative visual outcomes due to myopic retinal degeneration in 12 eyes of 8 patients (27.9%)
DISCUSSION
Nuclear subcapsular cataracts are known to be more predominant in highly myopic eyes(4,5,8). In our study, the frequency of nuclear cataracts was significantly higher than that of other types of cataract.
Non-contact optical biometry using partial coherence laser interferometry (IOL Master) provides more accurate and reliable measurements of postoperative refraction compared with conventional applanation ultrasound techniques(9). However, IOL Master measurements are affected by pupil dilation, fixation instability due to macular degeneration, and causes of media opacity, such as corneal scars, dense cataracts, or vitreous hemorrhage(9,10), all of which may cause biometric errors.
Calculation of the IOL power is critical in obtaining good visual outcomes. Presence of posterior staphyloma may cause errors in the measurement of the axial length. Older individuals with posterior staphyloma are more susceptible to larger increases in the axial length, with studies suggesting that B-scan ultrasonography should be performed in these patients to avoid errors in axial length measurement(6,11). The increase in the hyperopic refractive error with the axial length results from the use of positive power IOL constants for both positive and negative power IOLs; therefore, optimization of IOL constants improves the accuracy of IOL power calculations(6,12,13). The SRK/T formula has been found to be more accurate than other formulas in determining the IOL power in highly myopic eyes. However, the Haigis formula has been shown to be the most accurate method for determining the IOL power in eyes with extremely long axial lengths(12,14,15).
The SRK/T formula was used to determine the IOL power in the present study. We were unable to determine the definite causes of biometric error in our patients. Pupils were undilated during measurements, no patients had mature cataracts, and no patients had corneal scars or vitreous hemorrhage as patients with these disorders were excluded from the study. The biometric errors observed in the present study may be due to the formula used, because we did not optimize the constants and due to fixation instability resulting from myopic macular degeneration. In addition, preoperative corneal astigmatism may have affected the postoperative refraction despite the low rate of preoperative corneal astigmatism observed in the present study. Hyperopic errors are generally observed in patients with long axial lengths (more than 30 mm); however, in our study, we observed similar myopic errors in patients with long axial lengths compared with the other 2 groups, with a statistically higher mean biometric error in patients with long axial lengths compared with the other groups.
Mild postoperative myopia is generally recommended for cataract patients with high myopia. Patients usually request mild postoperative myopia as they prefer to see near objects more clearly(16). In the present study, the majority of the patients (75%) requested mild postoperative myopia. However, axial length may continue to increase with age in patients with high myopia, resulting in greater degrees of myopia with increasing age(11). Therefore, postoperative targeting of emmetropia may be more appropriate, particularly in younger cases. However, patient preference should always be taken into account. In addition, multifocal IOL may provide an alternate option in such patients, provided myopic macular degeneration is not severe.
A number of risk factors for RRD following cataract surgery have been identified, including male sex, young age, race, increased axial length, history of RD, non-intact posterior capsule, vitreous loss, vitreoretinal degenerations, and postoperative Nd:YAG laser capsulotomy(17-19). High myopia is the greatest risk factor for postoperative RRD(13,20,21). Intraoperative maintenance of an intact posterior capsule, IOL implantation, and anterior vitrectomy in cases of posterior capsular rupture are known to protect against RRD(22). It has been reported that the increased risk of RRD following cataract extraction may last for up to 2 decades following surgery(23). However, occurrence of RRD years after cataract surgery may be related to the natural history of myopia rather than pseudophakia.
Nd:YAG laser capsulotomy increases the risk of RRD owing to rupture of the anterior hyaloid membrane, liquid leakage from the anterior chamber, and vitreoretinal traction(24). In our study, 2 eyes developed retinal tears postoperatively and were treated with argon laser photocoagulation. Only 1 eye developed RD and the patient was referred to another hospital for retinal surgery. No patients developed RD following Nd:YAG laser capsulotomy.
Prophylactic treatment with argon laser photocoagulation for retinal pathologies prior to surgery is protective against RRD. However, retinal tears and RD can occur in previously normal retinal areas or at the edge of photocoagulation scars(7). The findings of the present study corroborate these previous results.
In conclusion, phacoemulsification surgery is associated with positive outcomes in patients with cataract and high myopia. However, the risk of postoperative retinal tears and RRD should be considered with prophylactic argon laser photocoagulation treatment used preoperatively where necessary.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

