INTRODUCTION
Trichosporon are saprophytic fungal species, which very rarely cause exogenous endophthalmitis and are possibly resistant to traditional antifungal therapy. Several endophthalmitis cases caused by Trichosporon species have been reported as a complication of fungemia in immunosuppressed patients and in one postoperative case(1,2). We present a case of postoperative endophthalmitis caused by amphotericin B-resistant Trichosporon asahii. To the best of our knowledge, this is the first report of T. asahiiendophthalmitis successfully treated with intravitreal and systemic voriconazole.
CASE REPORT
A 72-year-old man with a history of diabetes mellitus was admitted to our outpatient clinic with blurred vision, ocular pain, and redness in his left eye. He had undergone a cataract surgery on his left eye 1 month earlier at a different clinic. His symptoms had started 3 weeks after this surgery. His best-corrected visual acuities were 20/20 in the right eye along with perception of hand movements close to the face in the affected left eye. Slit-lamp biomicroscopy revealed a white flocculent mass with a moderate fibrin response and cellular reaction in the anterior chamber (Figure 1). Intraocular pressures measured by applanation tonometry were 16 mmHg in the right eye and 14 mmHg in the left eye. The fundus could not be visualized, and B-scan ultrasonography revealed vitreous opacities with an attached retina. Hence, the patient was diagnosed with postoperative endophthalmitis and was treated with intravitreal injections of vancomycin (1.0 mg/0.1 mL), ceftazidime (2.25 mg/0.1 mL), and amphotericin B (5 μg/0.1 mL), following a prompt vitreous tap and puncture of the anterior chamber to establish a culture. The patient then received topical fortified vancomycin (50 mg/mL), ceftazidime (100 mg/mL), amphotericin B (0.5 mg/mL), and dexamethasone every hour as well as oral moxifloxacin (400 mg) once daily. However, the patient’s vision deteriorated to the point where he was only able to perceive light, and the intraocular inflammation also worsened. Three samples from the initial vitreous tap were positive for Trichosporon species. All Trichosporon isolates were identified by standard laboratory procedures (morphological identification) and the VITEK 2 (bioMerieux, France) automated system. All three isolates were identified as T. asahii (Figure 2). Therefore, systemic amphotericin B (400 mg once daily) was added to the existing treatment, and a 23-gauge pars plana vitrectomy with silicon oil infusion and intravitreal injections of amphotericin B, vancomycin, and ceftazidime was performed 18 days post admission. Cultures that obtained during vitrectomy surgery again revealed T. asahii. Thus, the patient was administered another injection of intravitreal amphotericin B; however, the inflammation did not improve. For antifungal sensitivity tests, the minimum inhibitory concentrations (MIC) were determined using the reference broth microdilution method, according to Clinical and Laboratory Standards Institute document M27-A3. The MIC of fluconazole, voriconazole, amphotericin B, and caspofungin against the T. asahiiisolate were 2 μg/mL, 0.12 μg/mL, 2 μg/mL, and 4 μg/mL, respectively, demonstrating that the organism was resistant to amphotericin B but sensitive to voriconazole. Therefore, topical and systemic antifungal therapies were switched from amphotericin B to voriconazole (1 mg/mL topically), and intravitreal voriconazole (25 μg/0.1 mL) was also administered. Intravitreal voriconazole was repeated 3 days later. This therapy produced a significant clinical improvement with decreased intraocular inflammation, and it was discontinued after 8 weeks. Three months after the patient had been admitted, his vision was 20/100 with complete resolution of the endophthalmitis.
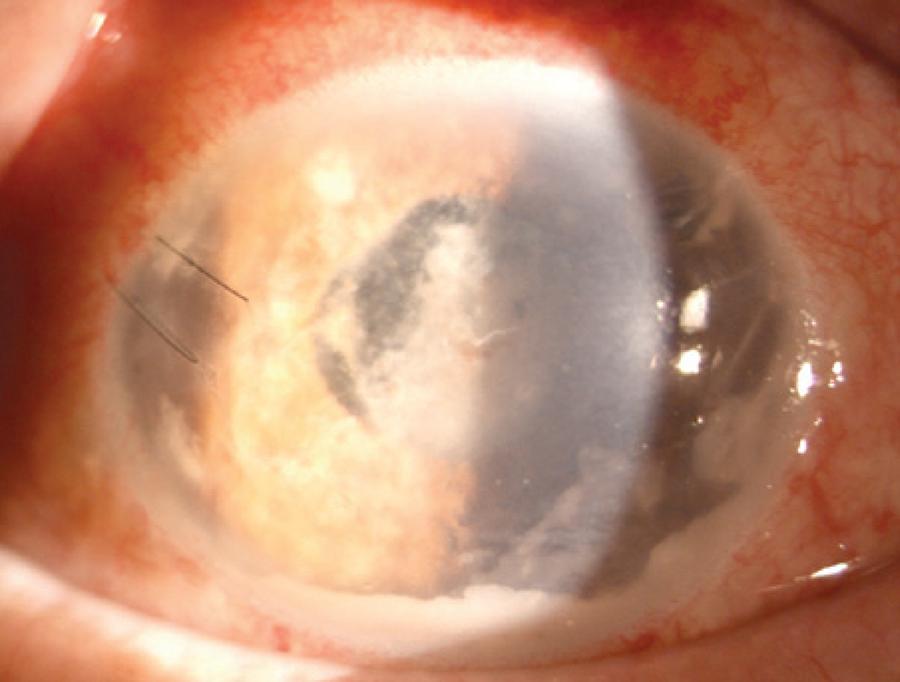
Figure 1 Slit-lamp photograph showing a white flocculent mass with a moderate fibrin response and cellular reaction in the anterior chamber.
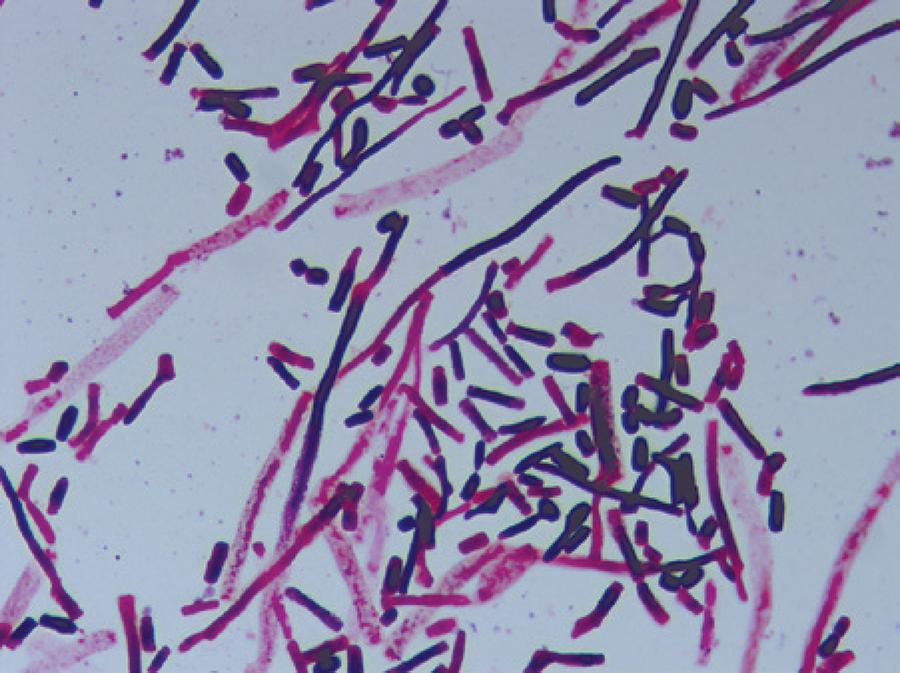
Figure 2 Photomicrograph of a Gram-stained colony isolated on Sabouraud dextrose agar, demonstrating budding yeasts, hyphae, and arthroconidia.
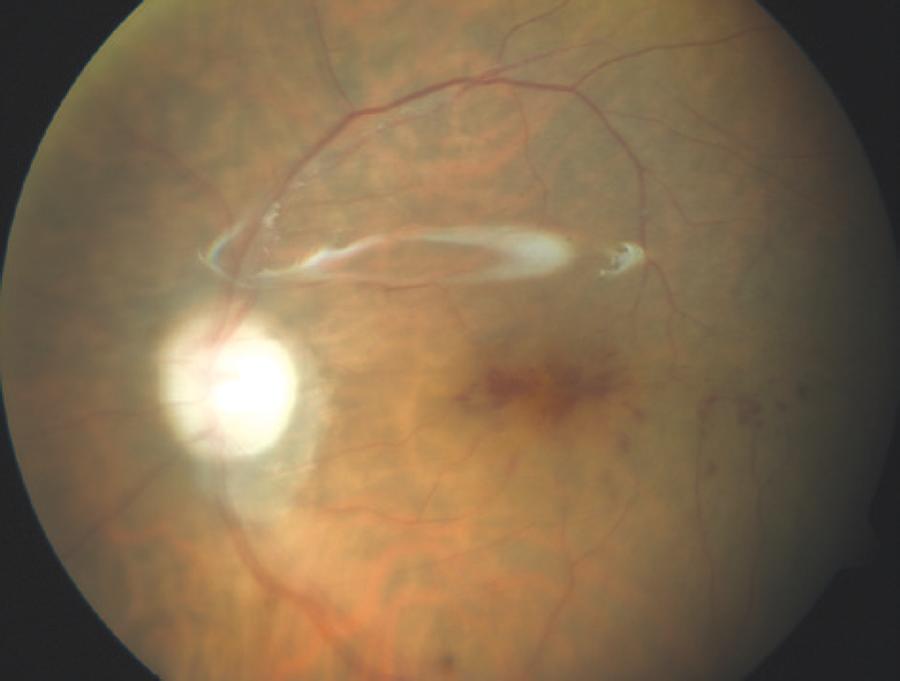
However, 6 months after the original cataract extraction, the infection recurred with a visual acuity of hand movements close to the face, and the patient underwent another pars plana vitrectomy with silicon oil infusion, removal of the intraocular lens and entire lens capsule, and intravitreal injection of voriconazole. Intravitreal voriconazole was also repeated 3 days after the second pars plana vitrectomy. Cultures of the vitreous and intraocular lens were positive for T. asahii.Therefore, systemic voriconazole was administered orally at a dosage of 200 mg twice daily for the next 2 months. Although inflammatory activity completely disappeared after this therapy, the patient’s visual acuity remained at 20/100 (Figure 3). No recurrence was observed at the 1-year follow-up.
DISCUSSION
Trichosporon species, formerly known as Trichosporon beigelii, rarely cause exogenous endophthalmitis and comprise a number of genetically distinct species that are pathogenic to humans(1,3,4). T. asahii is also among the Trichosporon species that are frequently observed in cases of immunocompromised patients(2-4). In addition, these species may exhibit some differences in their antifungal susceptibility profiles(3,4). Marc et al. reported a case of postsurgical endophthalmitis that was caused by T. beigelii in an immunocompetent patient with systemic and ocular sarcoidosis(1). In their case report, the patient was successfully treated with oral fluconazole. In addition, they postulated that sarcoidosis may have predisposed their patient to fungal endophthalmitis because patients with this disease may have a form of immune dysfunction. Similarly, our patient had no other immunocompromising risk factors, except for diabetes mellitus, which is associated with impaired leukocyte function(5). Therefore, diabetes mellitus may have predisposed our patient to T. asahiiendophthalmitis.
In vitro studies have shown that voriconazole is very effective against several fungal species that are resistant to the traditional antifungal therapy(3). In addition, some case reports demonstrate the successful use of systemic voriconazole in treating fungal endophthalmitis(2,6). In addition, intravitreal voriconazole concentrations of up to 25 μg/mL cause no electroretinographic changes or histologic abnormalities in the rat retina(7). However, the use of voriconazole in treatment of Trichosporon endophthalmitis has been limited. To the best of our knowledge, it has never been used to treat postsurgical fungal endophthalmitis caused by T. asahii. Moreover, voriconazole is reportedly the most effective agent against Trichosporon species in vitro(3,4). In addition, in our patient, the pathogen was resistant to amphotericin B. Therefore, we used intravitreal and systemic voriconazole. Furthermore, systemic or topical voriconazole should be ideally administered as follow-up to intravitreal voriconazole because voriconazole is rapidly metabolized after a single intravitreal injection(8). Hence, we used topical and systemic voriconazole after both the first and second pars plana vitrectomies.
Trichosporon species have the ability to adhere and form biofilms on implanted devices(4) such as intraocular lenses (IOL). This ability has been associated with infections by invasive Trichosporon species because these pathogens evade host immune responses and anti-fungal agents(4). In our case, the patient also had an IOL. Therefore, the ability of T. asahii to form biofilms on IOL may account for the occurrence and progress of the endophthalmitis in our patient.
In our case, recurrence after complete resolution of the endophthalmitis was observed. However, because we had not obtained the patient’s consent, we were unable to remove the IOL and entire lens capsule, both of which could be a reservoir of organisms, in the first pars plana vitrectomy; the recurrence was probably attributable to this. Although intravitreal and systemic voriconazole was used after the first vitrectomy, recurrence was observed 2 months after complete resolution of the endophthalmitis. In addition, after we used intravitreal and systemic voriconazole along with the removal of the IOL and entire lens capsule during the second pars plana vitrectomy, no recurrence was observed. Therefore, a possible reason for the recurrence in our patient was the biofilm of T. asahii that may have formed on the IOL.
In conclusion, voriconazole with intraocular debridement enabled successful resolution of the infection when treating endophthalmitis caused by amphotericin B-resistant T. asahii, demonstrating the importance of the removal of the IOL and entire lens capsule and also of injecting voriconazole intravitreally.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

