INTRODUCTION
The epithelium of the limbus and cornea ensure the functional and anatomical stability of the normal ocular surface of the human eye(1). Ridges in the conjunctival epithelium toward the peripheral corneal epithelium can be noted in the corneoscleral limbus, which were first described in 1921, and are now known as the palisades of Vogt(2). Fifty years later, Davanger and Evensen suggested that these ridges harbor corneal epithelial precursor cells(3).
In 1983, Thoft and Friend put forth a theory, known as XYZ, which determined the role of limbal stem cells in corneal epithelium renewal and maintenance(4). The corneal epithelium is in a state of constant renewal to ensure ocular surface maintenance, and this homeostasis depends on limbal stem cells.
Some situations, including thermal and chemical burns, autoimmune diseases, limbal failure secondary to contact lenses use, cryotherapy, or radiation, may alter or reduce the ability of the limbus to serve as a source of stem cells. In the wake of limbal stem cell deficiency, chronic epithelial defect, recurrent corneal erosion, neovascularization, chronic inflammation, fibrosis, and squamous metaplasia may occur(5).
Currently, the most common surgical technique to restore normal limbal physiology is limbal stem cell transplantation (LSCT)(5). The donor tissue can be taken from the same eye (ipsilateral conjunctival limbal autograft [CLAU]), the contralateral eye (contralateral CLAU), from a human leukocyte antigen (HLA)-matched relative (living-related conjunctival limbal allograft [lr-CLAL]) or from a cadaver (c-CLAL), depending on the severity and unilaterality or bilaterality of the clinical condition(6).
The purpose of this study is to report on the experience of the Ophthalmologic Hospital of Sorocaba (HOS), a tertiary teaching hospital, in performing these procedures, as well as the success and failure rates and associated complications.
METHODS
This is a retrospective study conducted between January 2003 and March 2008. During this period, 30 LSCT procedures were performed.
This study was approved by the Research Ethics Committee of the HOS, and adhered to the recommendations of the Declaration of Helsinki.
Any patient undergoing LSCT during the time of the study, regardless of the cause of limbal failure, patient age, or severity, was included in the study. Patients who did not undergo sufficient postoperative follow-up to determine the failure or success of the procedure were excluded. Two patients were excluded. Cases involving autologous grafts were classified as group I, and those involving allogenic grafts as group II.
A fellow surgeon assisted by an experienced cornea surgeon performed all the surgical procedures. All surgeries were performed under peribulbar anesthesia with 2% lidocaine. In group I, two fragments of the limbus and bulbar conjunctiva were obtained from the upper and lower regions of the healthy contralateral eye (CLAU). In group II, the grafts were obtained either from a living-related donor (lr-CLAL) after human leukocyte antigen I and II typing and cross-matching or from cadaver donor tissue (c-CLAL). In the recipient eye, a complete conjunctival peritomy was performed, followed by a lamellar keratectomy to remove fibrous pannus from the cornea. The limbal fragments were attached to the upper and lower limbus using a 10-0 monofilament nylon suture (Figure 1). In cases in which the amniotic membrane (AM) was used, it was placed either over or under the graft, or both over and under ("sandwich" technique).
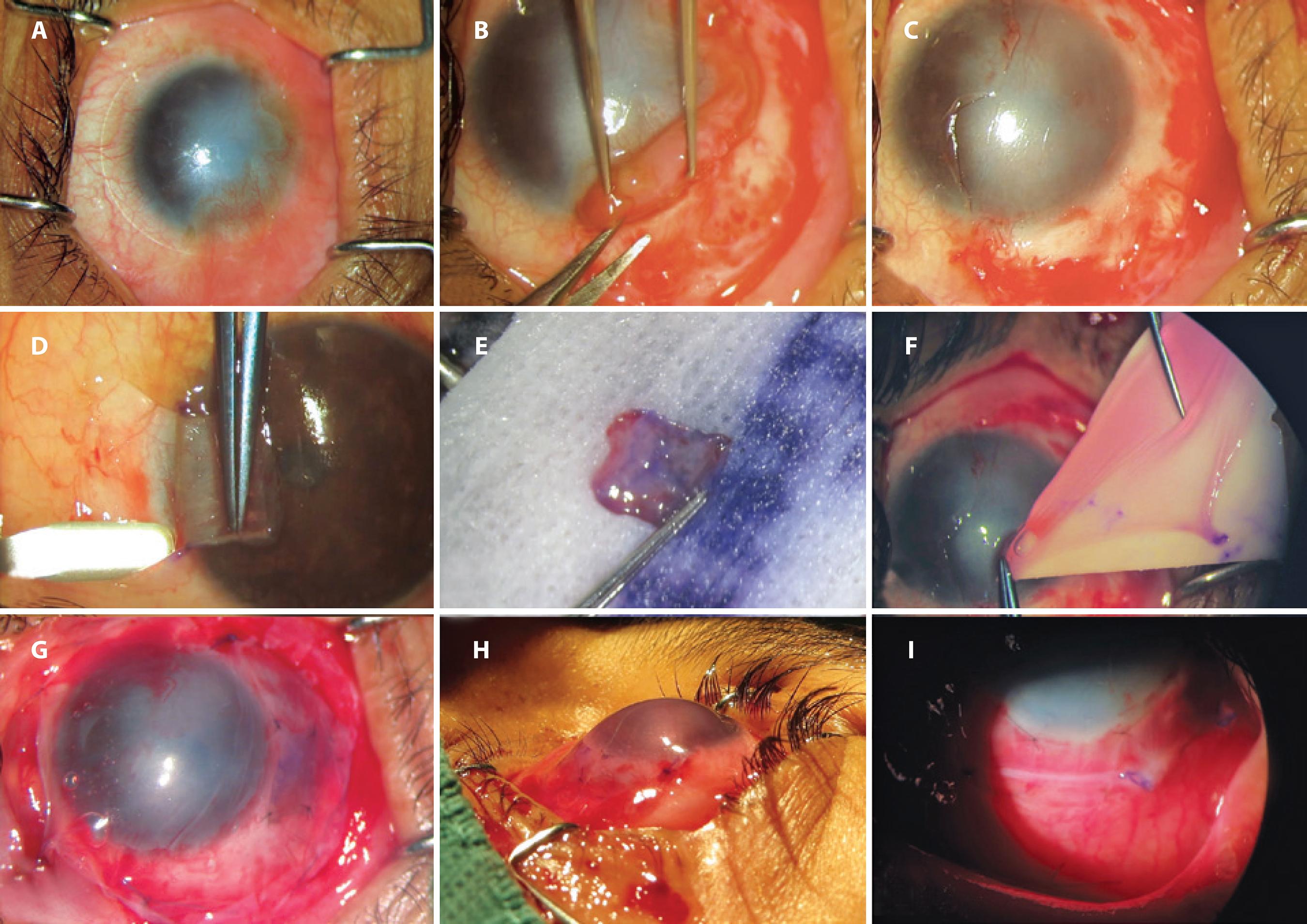
Figure 1 Surgical steps in a case of homologous limbal stem cell transplantation. A) preoperative photograph of a case of unilateral limbal stem cell deficiency; B) removal of fibrovascular tissue from corneal and scleral surfaces; C) underlying corneal and scleral bed preparation; D) autologous limbal tissue dissected from contralateral healthy eye at 6 o’clock position; E) autologous limbal tissue ready for transplantation; F) amniotic membrane transplantation; G) autologous limbal tissue sutured in place at 6 o’clock position under amniotic membrane transplantation (epithelial face down); H) immediate postoperative photograph; I) postoperative photograph on the seventh day.
The postoperative regimen was not standardized but all cases included corticosteroids and antibiotic eye drops (minimum of 4 weeks and 2 weeks, respectively) and preservative-free artificial tears. Systemic immunosuppression was implemented as a standard procedure in cases of c-CLAL and in lr-CLAL in which histocompatibility was not 100%. Systemic immunosuppression regimen and management were determined by a referred nephrologist and were not available in the medical records.
The data analyzed in this study included: gender, age, operated eye, condition causing limbal failure, time elapsed from the onset of the disease until surgery, unilaterality or bilaterality, autologous or allogenic transplantation, kinship of the donor in the case of allogenic transplantation, limbal extension, use of AM and positioning of its epithelial face, combination with tarsorrhaphy, duration of postoperative follow-up, probable cause of failure, uncorrected visual acuity before and after surgery, persistent epithelial defect or conjunctivalization of the cornea after surgery, improvement of corneal transparency, and postoperative complications.
In cases where persistent epithelial defect or conjunctivalization was not detected during the postoperative follow-up, the intervention was defined as a surgical success. All biomicroscopic findings reported that suggested failure, such as ischemia, melting, presumed rejection, necrosis, epithelial defect, or conjunctivalization were defined as failures.
Kaplan-Meier survival analysis was performed to evaluate conjunctival limbal graft survival.
RESULTS
The preoperative evaluation was performed for all 28 patients, from groups I and II, included in the study (Table 1).
Table 1 Epidemiological characteristics of the study population
| Epidemiological characteristics | Group I (n=15) autologous transplantation (CLAU) | Group II (n=13) allogenic transplantation (CLAL) |
|---|---|---|
| Age (years) | 48.83 (29-82) | 31.83 (5-63) |
| Sex (male/female) | 11/4 | 8/5 |
| Bilaterality | 2 (13.3%) | 9 (69.2%) |
| Mean time until surgery (years) | 9.61 | 12.62 |
| Limbal extension used | ≤120° (100%) | ≥120° (87.5%) |
| Use of amniotic membrane | 12 (80.0%) | 9 (69.2%) |
| Tarsorrhaphy | 6 (40.0%) | 10 (76.9%) |
| Mean postoperative follow-up (months) | 18.36 | 35.04 |
Of the total sample of 28 patients, 53.6% (n=15) underwent a CLAU and constituted group I, whereas 46.4% (n=13) underwent a CLAL and constituted group II.
Males were predominant in both groups (Table 1), and consequently, constituted the majority of the overall population studied: 67.9% (n=19). Right eyes were the most prevalent in the overall sample, at 67.9% (n=19), with 73.3% (n=11) in group I and 61.5% (n=8) in group II.
In relation to patient age at the time of surgical intervention, the mean age was 40.33 years (5-82 years). A higher mean age was found in group I, as seen in Table 1.
The groups differed with respect to bilaterality. Unilateral cases predominated in group I and bilateral cases predominated in group II as shown in table 1. In the overall analysis, unilateral cases accounted for 60.7% (n=17) and bilateral for 39.2% (n=11).
The most frequent pathology causing limbal stem cell deficiency among the entire study population was chemical burns (53%), followed by thermal burns (20.0%, n=3) and failure secondary to multiple surgical procedures (13.3%, n=2) in group I, and by Stevens-Johnson syndrome (23.0%, n=3), and atrophy, aniridia, and brachytherapy (each corresponding to 7.6%, n=1) in group II.
The duration of time of disease progression from diagnosis until surgery ranged from 1 month to 52 years, with an average of 11.18 years (Table 1).
The extension of donor limbal tissue transplanted was evaluated and the results are shown in table 1. The limbal tissue extension most used in the study was 120°, at 31.2% (n=5).
The groups were evaluated regarding the use of AM during surgery, as well as the positioning of its epithelial face. AM was used in the majority of the patients in the study: 75.0% (n=21); with 80.0% (n=12) in group I and 69.2% (n=9) in group II. In group I, the medical records of four cases did not contain information about the positioning of the membrane. For the rest of the cases, the membrane was positioned with the epithelial face downward (functioning as a contact lens) in four cases and upward (functioning as a basement membrane to stimulate epithelialization) in three cases. The "sandwich" technique (membrane facing both upward and downward) was used in only one patient in group I. In group II, there was no information about the positioning of the membrane in five cases; upward-facing positioning was used in three cases and downward in only one case.
Tarsorrhaphy and LSCT were common in group II (Table 1); this procedure was performed in 57.1% (n=16) of all cases.
With respect to the limbus donated for allogenic transplants, most came from a family member (lr-CLAL, 66.6%, n=8), and in all cases, the relative was a sibling. The c-CLAL accounted for 33.3% (n=4) of surgeries in group II. HLA compatibility analysis was performed in all patients in group II, but data was not available in the medical records. As a standard procedure, a minimum 75% match is required for an lr-CLAL.
Follow-up time ranged from 1 to 60 months, with an average of 24.84 months. Only one patient (group I) had a follow-up of 1 month due to early failure of the graft and did not return to continue treatment. This patient was included as a failure in the statistical analysis, and the complication was reported. All other patients in the study had a minimum follow-up of 6 months. The mean follow-up time for each group is found in table 1.
There was improvement in the uncorrected visual acuity in 38% of all cases (n=8), no change in 28.5% (n=6), and deterioration in 33.3% (n=7).
The postoperative results were obtained from the medical records, with a focus on persistent epithelial defect, conjunctivalization, and improved transparency. No persistent epithelial defect was found in the majority (75.0% of the total study population; n=21) of the patients in both groups as depicted in table 2. The conjunctivalization rate was similar between the two groups, at 53.3% (n=8) in group I and 58.3% (n=7) in group II. Improved transparency occurred in only 38.4% (n=10) of the cases, corresponding to 46.6% (n=7) of the cases in group I and 23.07% (n=3) of the cases in group II.
Table 2 Postoperative results: complications and success rates
| Results | Group I (n=15) | Group II (n=13) | Total (n=28) |
|---|---|---|---|
| Persistent epithelial defect | 2 (13.30%) | 5 (38.4%) | 7 (25.0%) |
| Melting | 1 (6.60%) | 3 (23.0%) | 4 (14.2%) |
| Graft necrosis or ischemia | 0 | 2 (15.3%) | 2 ( 7.1%) |
| Descemetocele | 0 | 2 (15.3%) | 2 ( 7.1%) |
| Infectious ulcer | 0 | 1 ( 7.6%) | 1 ( 3.5%) |
| Perforation | 0 | 1 ( 7.6%) | 1 ( 3.5%) |
| Surgical success | 5 (33.00%) | 3 (23.0%) | 8 (28.5%) |
| Time to surgical failure (months) | 2.16 | 2.25 | 2.21 |
Surgery was considered successful when there were no reports of persistent epithelial defect or conjunctivalization during the postoperative follow-up. Any biomicroscopic signs suggesting failure of the graft, such as ischemia, melting, presumed rejection, necrosis, epithelial defect, or conjunctivalization, were considered as surgical failures. Using these criteria, the success rate was eight out of 28 patients, which constitutes 28.5% of all surgeries performed. The LSCT success rates in groups I and II can be seen in table 2.
Among those cases considered to be failures, the average time between surgery and diagnosis of failure was approximately 2 months, and was similar between the two groups (Table 2).
Kaplan-Meier survival analysis was applied to evaluate conjunctival limbal graft survival. In group I, graft survival was seen in six eyes (43%) at 1 year and in three eyes (21%) at 3 years, with a cumulative survival of 21% after a mean follow-up time of 18.36 months. In group II, survival was seen in three eyes (38%) at 1 year and in two eyes (15%) at 3 years, with a cumulative survival of 15% after a mean follow-up time of 35.04 months (Figure 2).
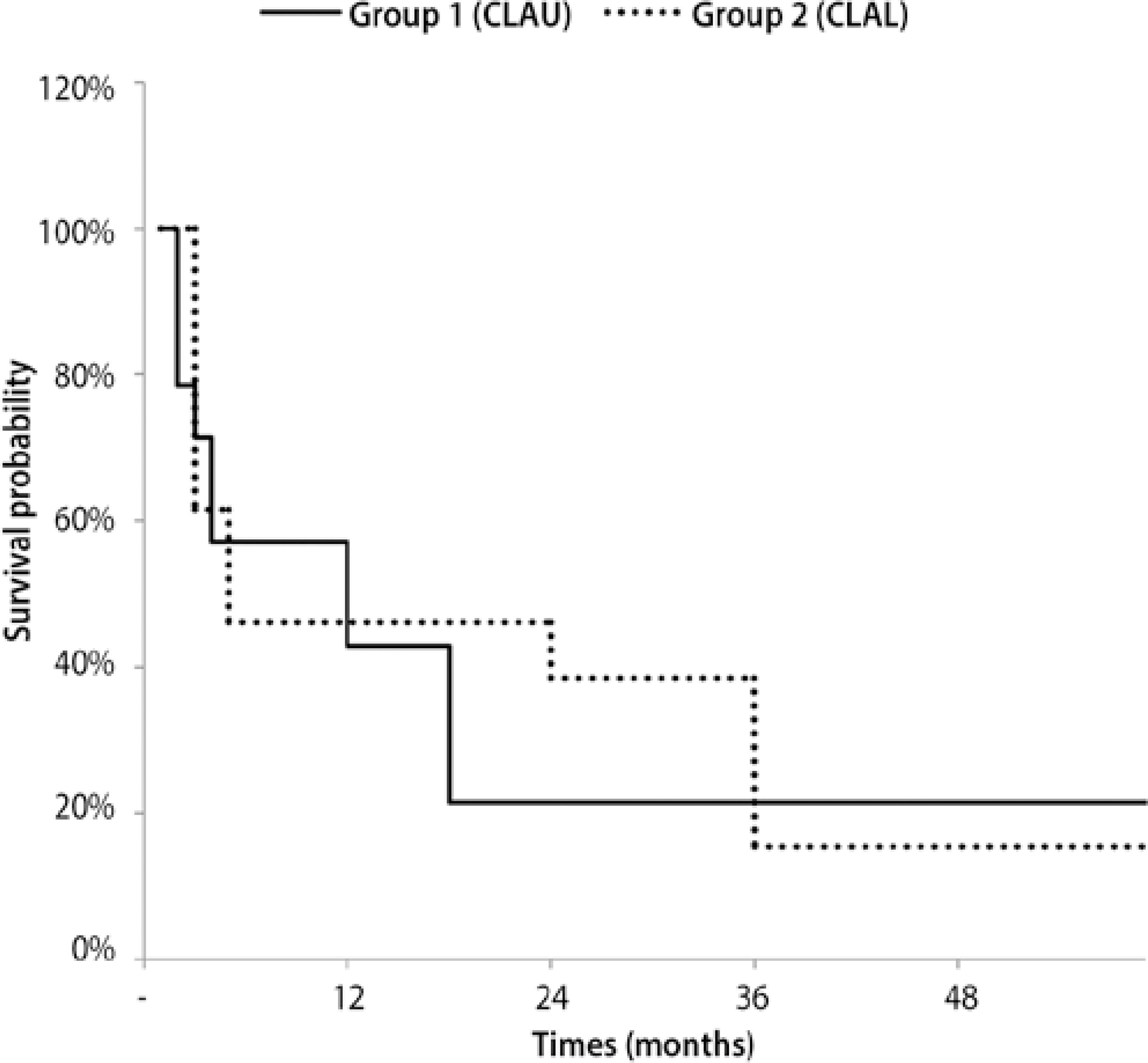
Figure 2 Kaplan-Meier survival curve of autologous grafts (group I) and allogenic grafts (group II).
The most prevalent complication was persistent epithelial defect, which occurred in 25.0% of the cases (n=7), followed by melting in 14.2% (n=4). The other complications observed include infectious ulcers, limbal graft necrosis or ischemia, perforation, and descemetocele; their prevalence in each group is shown in table 2.
DISCUSSION
Many studies have already reported that limbal stem cells are the source of epithelial renewal and regeneration under normal or injury conditions(3).
The replacement of limbal stem cells through LSCT, is one of the most effective methods for treating limbal stem cell deficiency(6,7). The technique is chosen depending on the extent and severity of damage to the limbal function and the clinical conditions of the contralateral eye. Therefore, in situations where it is unilateral and unifocal, an ipsilateral CLAU can be performed. When the unilateral damage is more extensive, the contralateral limbus can be used (contralateral CLAU). On the other hand, for bilateral situations, the limbus of close relatives or a cadaver is used(6). For CLAL, HLA typing analysis is necessary and the use of systemic immunosuppression is considered(6,7).
In this study, the male sex (67.9%) and right eye were more commonly affected. A previous small series has also reported a higher prevalence among males(8). Since the main cause of limbal stem cell deficiency in this study was chemical injury, we believe that the higher incidence in males may be related to greater occupational exposure in males.
The distribution of patients in groups I and II took into account the severity and unilaterality or bilaterality. This fact limits comparisons between the two groups insofar as the success and failure rates of the treatments applied, since it involved patients with different clinical profiles who also underwent distinct surgical procedures. Success rate analysis should also take into consideration the technical inexperience of surgeons who were still learning.
Only 13.3% (n=2) of the 15 patients in group I had bilateral disease, whereas 69.2% of the cases in group II had bilateral involvement (n=9). In the two cases where there was bilateral disease and CLAU was performed, the patients had partial and localized limbal failure in the least affected eye caused by chemical burns. In group II, four patients had unilateral disease (30.8%), and despite this, underwent CLAL. Three of them had previously undergone unsuccessful CLAU and one had a contralateral eye in phthisis bulbi.
The literature on the subject indicates that chemical injury is largely responsible for most cases of limbal failure(7), which we also found to be the case in our study (n=15; 53.5%). Other causes included thermal burns (n=3; 10.7%) and Stevens-Johnson syndrome (n=3; 10.7%). Santos et al. evaluated 33 eyes with limbal stem cell deficiency, and of these, 67% were secondary to chemical trauma and 33% due to Stevens-Johnson syndrome(9). In an extensive literature review, Cauchi et al. reported that 50% of the cases of bilateral limbal stem cell deficiency were caused by chemical burns, whereas this same etiology was responsible for 100% of the cases of unilateral limbal stem cell deficiency(10).
The use of AM has already been well documented in the treatment of cutaneous ulcers(11-14). It was first used in ophthalmology in 1940 to treat conjunctival defects(15-17). AM works in a therapeutic manner, with specific properties that promote ocular surface repair, such as anti-adhesive and antibacterial effects, wound protection, pain reduction, and especially, in promoting reepithelialization(11-14,18,19). It can also serve as a barrier against fibroblast proliferation and exhibits low immunogenicity due to not exhibiting higher histocompatibility antigens (HLA-A, B, or DR), which prevents rejection in the host(20).
Some encouraging results have been published in terms of ocular surface reconstruction using AM(7). Its use may provide a more suitable environment for cell proliferation than the previously inflamed perilimbal environment, thus increasing the chances of successful ocular surface reconstruction(7). In this study, AM was used in 75% of the cases.
Another important contributing factor in corneal epithelialization in ocular surface reconstruction surgeries is tarsorrhaphy(21). Besides reducing tear film evaporation, it also reduces the traumatic effect caused by blinking(21). Tarsorrhaphy was used in conjunction with LSCT in 57% of the overall population in the study and was performed in group II in 77% of the procedures. Since group II was comprised of patients with greater clinical severity, and for the most part, with bilateral conditions, this explains the higher use of tarsorrhaphy as an adjunct in this group of patients.
The definition of surgical success in studies on limbal stem cell deficiency varies in the literature, but it mostly includes reepithelialization and visual results, which respectively indicate anatomical and functional recovery of the ocular surface(22-24).
Shimazaki et al. used the detection of donor tissue-derived epithelial cells in patients who underwent heterologous LSCT combined with penetrating keratoplasty, comparing with isolated cases of penetrating keratoplasty as controls. This study suggested the long-term effectiveness of CLAL in providing epithelial cells from tissue transplanted to the ocular surface(24).
Ilari et al. studied 23 eyes with severe ocular surface disorders that underwent CLAL, and evaluated the reepithelialization and stabilization of the whole epithelium over a mean follow-up period of 60 months. They detected primary failure (eyes that never reepithelized) in 24.2% of the cases. Only 21.2% of the cases retained an intact epithelium throughout the follow-up(25).
In another study that evaluated the results of LSCT combined with penetrating keratoplasty, Reinhard et al. detected central transparency in 30% of the grafts after 5 years of postoperative follow-up. Additionally, there was an improvement in visual acuity from 0.02 to 0.24 in patients with a clear graft and from 0.02 to 0.03 in cases of failure. The best visual acuity observed after long-term follow-up was 0.45 in patients with a clear graft and 0.26 in cases of failure(23).
Solomon et al. evaluated 39 eyes that underwent CLAL with AM, with or without penetrating keratoplasty, and found that the overall survival of ambulatory vision (deemed as visual acuity greater than 0.1) was 53.6% at 3 years and 44.6% at 5 years(26).
A retrospective study such as the present study tends to obtain visual acuity as measured using a Snellen chart by different examiners and/or environments with non-standardized lighting. For this reason, visual acuity was not included as a criterion for evaluating surgical success. In this study, the absence of epithelial defects and conjunctivalization during postoperative follow-up were considered as variables indicating surgical success and totaled 28.5% (n=8) of the sample population. The success rate in groups l and II were 33.0% (n=5) and 23.0% (n=3), respectively. Again, it is worth noting the inclusion of patients with more severe and mostly bilateral clinical profiles in group II, requiring allogenic transplantation, which prevents the comparison of the success rates between the two groups.
The length of follow-up time may be directly related to the survival rate of limbal grafts. Santos et al. found a cumulative survival rate of 80% for CLAU and 13% for CLAL after 33 months of follow-up(9). Solomon et al. noted a progressive decrease in the survival rates of keratolimbal allogenic grafts over 5 years of follow-up, with 76.9% at 1 year, 47.4% at 3 years, and 23.7% at 5 years(26). This study also found a better survival rate when LSCT was performed as an isolated procedure, as opposed to the procedure being combined with penetrating keratoplasty, with rates of 81.2% for the former and 58.9% for the latter at 2 years. Furthermore, when necessary, a limbal retransplantation had a higher survival rate at 2 years than the first procedure, with rates of 68.2% and 27.3%, respectively (p=0.041). In a study of 23 eyes with severe ocular surface disorders that underwent CLAL, Ilari et al. found survival rates of 54.4% at 1 year, 33.3% at 2 years, and 27.3% at 3 years(25).
The mean follow-up time of patients in this study was 24.84 months; a longer follow-up period would be required to estimate the long-term success rate of these patients.
The survival rate can also be influenced by HLA compatibility in cases of CLAL. Reinhard et al. found a variable survival rate at 5 years that was dependent on HLA compatibility, at 65% for incompatibility of 0-1 alleles, 41% for incompatibility of 2-6 alleles, and 14% for total incompatibility of alleles (p=0.03)(23). In this study, the donor tissue was from a family member (66.6%, n=8) for the majority of the allogenic transplants, and the limbus taken from cadavers accounted for 33.3% (n=4). HLA compatibility analysis was performed for all patients in group II, but the data was not available in the medical records. As a standard procedure, a minimum of 75% class I and II HLA match is required for an lr-CLAL.
Despite the variety of surgical procedures for ocular surface restoration, the success rate of current techniques decreases over time, ranging from 40-80% during the first year to 33-50% over 2 years(8,9,27). Taking into account the relatively low medium-term success rates, it is worth considering alternative therapies, such as keratoprosthesis implantation. The anatomic and functional results reported in the literature support the use of Boston type 1 keratoprosthesis for managing bilateral limbal stem cell dysfunction secondary to chemical burn(28,29). Another interesting option is the combination of regenerative medicine based on regenerative cell therapy (isolation and transfer of undifferentiated cells)(30). Cell therapy, consisting of undifferentiated stem cells, can be genetically modified before they are transplanted to the recipient, which would not only offer a regenerative therapeutic alternative, but at the same time, provide a lasting and stable genic presence of therapeutic genes in the pro-genesis of these cells.
Despite this unfavorable long-term prognosis, LSCT still provides acceptable results in developing countries through a relatively simple, low-cost procedure, and continues to be a therapeutic alternative for cases of unilateral or bilateral limbal stem cell deficiency.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

