INTRODUCTION
Preterm birth is a significant public health concern, as it is associated with a high risk of infant mortality, various morbidities in both the neonatal period and later age, and significant socio-economic difficulties(1,2). Prematurely born children are disadvantaged in terms of perinatal mortality and long-term growth(3-5), and they have low birth weight and shorter eyes as compared with full-term children(6,7).
At present, ultrasound and optical biometry (partial coherence laser interferometry) are used to measure intraocular distances. However, eye measurements in newborns are only possible using ultrasonic methods. Ultrasound biometry, commonly referred to as A-scan and B-scan, is utilized for diagnostic testing and biometric measurements(8). A-scan ultrasonography provides a one-dimensional measurement of length in the axial plane. Additionally, it facilitates the monitoring eye growth during infancy(9). The ultrasound axial length of the eye is measured using either contact or immersion techniques. The contact technique is used more frequently, while measuring the axial length of children’s eyes by pediatric cataract surgeons(10).
This study is aimed to measure ocular biometric parameters in premature infants and to investigate their relationship with birth weight, gestational age, and postmenstrual age.
METHODS
Infants
Premature infants enrolled in the retinopathy of prematurity (ROP) screening and who were born between September 1, 2013 and January 1, 2014 at Zekai Tahir Burak Women’s Health Education and Research Hospital were selected for this cohort study. The inclusion criterion was birth at ≤36th gestation week. Infants with all types of congenital anomalies were excluded, even mild ones, and those with congenital eye abnormalities were also excluded from the study. Infants who had received previous treatment, such as laser photocoagulation and/or intravitreal injections, were also excluded.
Examinations for ROP and biometry measurement were initiated between postnatal weeks 4 and 5. Follow-up examinations were planned at approximately 1-3-week intervals depending on ROP results and retinal findings. The measurements were performed at approximately one-month intervals. Biometry measurements were performed on both eyes, but only data from the right eye were included in the analysis.
This study was approved by the local ethics committee of Zekai Tahir Burak Women’s Health Education and Research Hospital and performed in accordance with the ethical standards stipulated in the Declaration of Helsinki. Parents or guardians of all infants gave informed consent prior to the examinations.
Birth parameters
Data on birth weight (g) and gestational age (weeks) were obtained from medical records, the hospital physician, or nurse records. The birth weight of a newborn was measured using an electronic weighing machine. Newborns were weighed without clothes within the first few hours of delivery. Gestational age was determined on the basis of the first day of the last normal menstrual period and the day of delivery, or on the basis of prenatal ultrasonography(11).
Eye examinations and measurements
Topical phenylephrine hydrochloride 2.5% with topical tropicamide 1% were administered two times at an interval of 10 min, and fundoscopy was performed only after a minimum of 30 min after the latter administration(12). The eyelids were retracted using a pediatric speculum following the administration of the topical anesthetic 0.5%-proparacaine hydrochloride. An indirect ophthalmoscope (Heine Optotechnik, Herrsching, Germany) was used for fundoscopy with scleral indentation. ROP was graded according to The International Classification of Retinopathy of Prematurity(13).
Anterior chamber depth, lens thickness, vitreous length, and axial length were measured with an A-scan biometer (Compact Touch 3-in-1 Ultrasound system, B-scan, Biometry, Pachymetry; Cedex, France). The A-scan probe was placed gently on the center of the cornea, perpendicular to its axis. Researchers were careful to avoid the indentation of the cornea. The average value of at least five measurements was recorded for each eye.
Data analysis
Statistical analysis was conducted using Statistical Package for the Social Sciences™ 16.0 (SPSS Inc. Chicago, IL). Results are reported as means ± standard deviation. The one-sample Kolmogorov-Smirnov test was used for determining normally distributed variables, and one-way analysis of variance (ANOVA) test was used for evaluating the homogeneity of variance.
For comparison of gender with biometry parameters (anterior chamber depth, lens thickness, vitreous length, and axial length), the measurements during the first examination were evaluated, and a parametric test, t-test, for independent samples, was used. The Pear-son product-moment correlation coefficient was used to evaluate the relationship of biometry parameters with birth weight and gestational age during the first examination and with postmenstrual age at all examinations. Differences were considered significant at a probability (p) level <0.05, and correlation coefficients were considered to be significant at r>0.5.
RESULTS
The study population comprised 185 females and 176 males, adding up to a total of 361 infants. Gestational age and birth weight values ranged from 23 to 36 weeks and from 560 to 2,670 g, respectively. The mean gestational age and birth weight were 30.8 ± 2.8 weeks and 1,497.9 ± 483.6 g, respectively. During the follow-up period, 159 (approximately 44.0%) infants developed ROP, whereas 202 (approximately 56.0%) did not. Stage 1, stage 2 and stage 3 ROP were developed by 95 (59.7%), 48 (30.1%), and 16 (10.0%) infants, respectively. The first examination took place at the 4th or 5th postnatal week. Data from at least one examination for each infant was used in the study. The mean number of examinations was 2.6 per infant, yielding a total of 939 examinations.
Mean birth weight and gestational age demonstrated no significant difference between girls and boys at the initial examination (p>0.05) (Table 1). In male infants, the mean anterior chamber depth was 0.10 mm longer, the vitreous length was 0.06 mm longer, and axial length was 0.09 mm longer, while the mean lens thickness was 0.05 mm shorter as compare with the female infants. However, female and male preterm infants did not differ significantly in any aspect of biometric parameters (p>0.05). The postmenstrual age of the infants ranged from 28 to 56 weeks during follow-up, and the mean postmenstrual age during the first examination was 35.2 ± 5.7 weeks (Table 2).
Table 1 Comparison of mean gestational age and mean birth weight among male and female infants
| Female | Male | p | Total | |
|---|---|---|---|---|
| Number | 185 | 176 | 0.172 a | 361 |
| Mean gestational age (weeks ± SD) | 30.7 ± 2.9 | 31.0 ± 2.7 | 0.124 b | 30.8 ± 2.8 |
| Mean birth weight (g ± SD) | 1,420.1 ± 477.0 | 1,576.3 ± 418.9 | 0.064 b | 1,497.9 ± 483.6 |
a= one-sample Kolmogorov-Smirnov test;
b= independent-sample t-test; SD=standard deviation; n= number; g= gram.
Table 2 Comparison of mean biometry parameters during the first examination among male and female infants by the t-test for independent samples
| Mean ± SD | Female (n=185) | Male (n=176) | p | Total |
|---|---|---|---|---|
| Postmenstrual age (week) | 34.90 ± 3.10 | 35.60 ± 3.40 | 0.124 | 35.20 ± 3.80 |
| Anterior chamber depth (mm) | 2.14 ± 0.28 | 2.24 ± 0.32 | 0.097 | 2.19 ± 0.36 |
| Lens thickness (mm) | 3.68 ± 0.64 | 3.61 ± 0.34 | 0.095 | 3.64 ± 0.53 |
| Vitreous length (mm) | 10.20 ± 1.52 | 10.26 ± 1.87 | 0.716 | 10.23 ± 1.04 |
| Axial length (mm) | 16.02 ± 1.05 | 16.11 ± 0.68 | 0.129 | 16.06 ± 0.73 |
SD= standard deviation; n= number; mm= millimeter.
The correlation between birth weight and gestational age with biometry parameters is shown in table 3. During the first examination, the mean postmenstrual age was 35.2 ± 3.8 weeks, and the birth weight and gestational age of the infants correlated significantly and positively with lens thickness, vitreous length, and axial length (r>0.5, p<0.001). However, no strong correlation was found between birth weight and gestational age with anterior chamber depth (r<0.5).
Table 3 Correlation between birth weight and gestational age with biometry parameters during the first examination
| Anterior chamber depth | Lens thickness | Vitreous length | Axial length | |
|---|---|---|---|---|
| Birth weight | r=0.037, | r=0.551, | r=0.612, | r=0.577, |
| p=0.775 | p<0.001 | p<0.001 | p<0.001 | |
| Gestational age | r=0.125, | r=0.582, | r=0.680, | r=0.634, |
| p=0.001 | p<0.001 | p<0.001 | p<0.001 |
(r= Pearson correlation).
Increased vitreous and axial lengths correlated significantly with increasing postmenstrual age of the infants (r=0.669, p<0.001; r=0.845, p<0.001, respectively). Anterior chamber depth and lens thickness increased with increasing postmenstrual age. However, the correlation between anterior chamber depth and lens thickness with postmenstrual age was weak (r=0.432, p<0.001; r=0.412, p<0.001, respectively) (Figures 1-4).
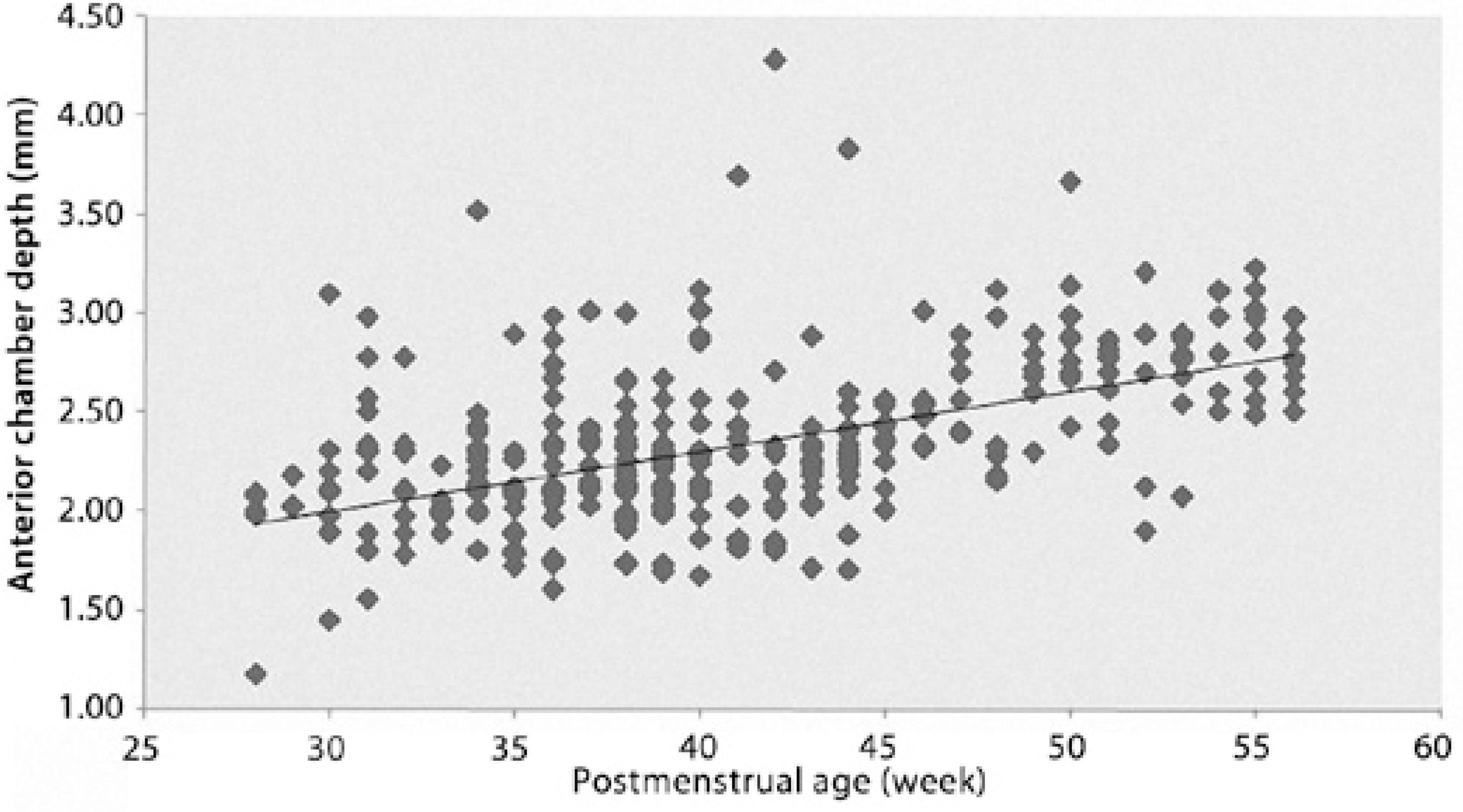
Figure 1 Relationship between anterior chamber depth and postmenstrual age (r=0.432, df=938, p<0.001). The regression line fit to the data has a slope of 0.029.

Figure 2 Relationship between lens thickness and postmenstrual age (r=0.412, df= 938, p<0.001). The regression line fit to the data has a slope of 0.039.
DISCUSSION
The eye undergoes significant growth between the neonatal period and adulthood. Investigations of the globe in neonates and infants have demonstrated that the posterior segment of the globe is relatively less developed than the anterior segment. These parameters change rapidly over the first 18 months of age(14-16). The A-mode of ultrasound (amplitude mode) is a type of ultrasound in which a single transducer scans a line through the body with echoes plotted on a screen as a function of depth. Generally, in children, A-scan biometry is used for measuring the anterior-posterior diameter of eye. Ultrasonography is commonly used for clinical examinations of infants because of its safety and non-invasive character(17).
The mean axial length of the full-term newborn eye is 16.8 mm, while in adults it is 23.6 mm(18). However, the axial length of the eye in term infants varies according to the method of measurement. Lengths obtained by ultrasonographic biometry tend to be shorter than the lengths obtained by pathologic studies. It was reported that the newborn eye had a mean axial length between 17.1 and 17.5 mm. At term, the anterior chamber depth averages 2.05 mm, with a range of 1.8-2.4 mm(19). Isenberg et al.(20) demonstrated that the mean axial length was 16.2 mm, the anterior chamber depth was 2.0 mm, lens thickness was 3.8 mm, and vitreous chamber depth was 10.5 mm for term newborns. Globe size and axial length undergo dramatic changes during infancy. Although the anterior chamber depth of a newborn eye is approximately 75%-80% of that of adult eyes, their posterior segment at birth is less than half the size of that of the adult eye(14). Similarly, in the present study, we found that the axial length elongation is mostly due to vitreous chamber elongation during the growth of postmenstrual age of the infants, i.e., at the age of 28th-56th week.
There are few reports in the literature on ocular biometric parameters in premature infants. Axial length continues to increase from birth. High refractive errors are common in the neonatal period following full-term and preterm birth and are related with poor emmetropization. It was argued that premature birth signals increased the risk of abnormal refractive development(21-24). Kobayashi et al.(25) measured anterior segments in 39 premature infants (at the gestational age of 25-39 weeks) using ultrasound biomicroscopy. They found that the mean anterior chamber depth was 1.3 mm at the 34.4 postconceptional week. These values appear quite low compared with our findings; we detected an anterior chamber depth of 2.10 mm at the 34th postmenstrual week (Figure 1).
We found that the mean anterior chamber depth was 2.19 mm, lens thickness was 3.64 mm, vitreous length was 10.23 mm, and axial length was 16.06 mm at the 35th postmenstrual week. Our results are in agreement with the investigation of Cook et al.(26) with regard to the development of biometric parameters in premature infants with or without retinopathy of prematurity (Figures 1-4). These authors obtained a mean axial length between 16.37 and 16.66 mm, anterior chamber depth between 2.14 and 2.26, posterior segment length between 10.18 and 10.47, and lens thickness between 3.93 and 4.04 mm. Similarly, we have previously reported the mean anterior chamber depth as 2.1 ± 0.4 mm, lens thickness as 4.1 ± 0.7 mm, vitreous length as 10.3 ± 1.5 mm, and axial length as 16.4 ± 1.3 mm in 138 eyes of 69 premature infants with ROP(27).
In the present study, the anterior chamber depth, vitreous length, and axial length were slightly greater in boys than girls. On the other hand, the lens was slightly thicker in girls than boys; however, these differences were insignificant. In a similar manner, the mean axial length in the male gender for term neonates was reported to be 0.2 mm longer than that for the female neonates(19). Laws et al.(28) also found that male infants had longer axial lengths. This may be related to larger biparietal or occipitofrontal head diameter and to heavier weight of male infants. In another study, axial growth was measured at 3 and 9 months of age with similar findings(15).
Both birth weight and gestational age have an effect on ocular growth(26). Saw et al.(29) examined the association of birth parameters with biometry in children aged 7-9 years, and suggested that children who were born heavier or who were born more mature had longer axial lengths and deeper vitreous chambers. However, they found no significant association between birth weight and lens thickness or anterior chamber depth. We documented that the birth weight and gestational age had a significant effect on lens thickness and vitreous and axial lengths. However, their impact on anterior chamber depth was minimal. As mentioned, infants with high gestational age and birth weight have larger head circumference and longer ocular biometric parameters.
The relationship between the size of the eyeball and other factors, such as birth weight, gestational age, and postmenstrual age has recently been documented(9,28-30). Axial length grows linearly during the postnatal period in premature infants(28). Axer-Siegel et al.(30) reported that in preterm infants with maturation, the anterior chamber depth and the axial length are enlarged, whereas lens thickness remains stable. In our study, both vitreous and axial lengths showed strongly positive correlation with postmenstrual age. However, anterior chamber depth and lens thickness showed little positive change in correlation with the growth in postmenstrual age.
It is important to clarify the role of birth parameters on ocular biometric measures, such as anterior chamber depth, lens thickness, vitreous length, and axial length, in premature infants. While the anterior chamber depth, vitreous length, and axial length were slightly longer in boys, the lens was slightly thicker in girls at the average 35th postmenstrual week. Birth weight and gestational age had a significant effect on lens thickness, vitreous length, and axial length but had little impact on anterior chamber depth in this study. Moreover, the vitreous length and axial length showed strongly positive correlation with postmenstrual age. However, anterior chamber depth and lens thickness showed little change in correlation with the growth in post-menstrual age.
In this study, there were several limitations, including a short study period, lack of a control group including full-term infants, and the fact that it is a single-center study. In conclusion, our study demonstrated that axial length elongation is mostly due to an increase of the posterior chamber length during the growth of premature infants in the postmenstrual 28th-56th week.





 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

