INTRODUCTION
Intravitreal injection of antivascular endothelial growth factor agents is widely used to treat several retinal diseases, including diabetic macular edema, macular edema secondary to retinal vein occlusions, and neovascular age-related macular degeneration( 1 , 2 ). Although past studies have demonstrated the relative safety of intravitreal injections( 3 , 4 ), the pain associated with injection can cause eye movements that may result in injection-related complications. Despite the numerous methods of local anesthesia used for intravitreal injections, including peribulbar and subconjunctival anesthetic injections, anesthetic eye drops, gels, and anesthetic soaked pledgets, there is no consensus on the best anesthetic option( 5 - 7 ). This study aimed to compare the effectiveness of topical proparacaine 0.5% drops, proparacaine plus subconjunctival lidocaine, and 2% lidocaine gel for anesthesia during intravitreal injections.
METHODS
This prospective, randomized, triple-armed trial compared the effectiveness of 3 different anesthetic approaches for intravitreal injections. The study included 92 consecutive patients scheduled to receive an intravitreal injection of bevacizumab (Avastin; Genentech, Inc.) in 1 eye, from June 2014 to September 2014 at the Retina Clinic, Osasco, São Paulo, Brazil. The same ophthalmologist (CGA) administered both anesthetic and therapeutic injections. A nurse masked to the treatment collected the patient assessment responses and a statistician masked to the treatment performed statistical analyses. The research followed the tenets of the Declaration of Helsinki and was approved by the institution’s Committee of Ethics in Research. All participants provided written informed consent prior to participation in the study.
Patients were randomized to 1 of 3 groups before injection: proparacaine 0.5% drops (Anestalcon®; Alcon, São Paulo, Brazil; Group Drops), proparacaine plus subconjunctival lidocaine 1% (Xylestesin®; Cristália, São Paulo, Brazil; Group SC), or 2% lidocaine gel (Xylestesin®; Cristália; Group Gel). The randomization scheme was generated using the web site Randomization.com (http://www.randomization.com). A physician masked to the treatment selected a sealed envelope, arranged in sequential order, containing the treatment randomization. The ophthalmologist (CGA) and patients were blinded to the allocation sequence. The order in which patients were recruited corresponded to the order in which they were scheduled in the clinic.
A standardized method was used to prepare the injection site and to disinfect the skin using povidone iodine 10%. Patients from groups Drops and SC received a drop of proparacaine 0.5% followed by a drop of povidone iodine 5%. For patients from Group Gel, the gel was placed on the eye before the drop of povidone iodine 5%. Patients from Group Drops received a second drop of proparacaine 0.5% 5 min after the drop of povidone iodine 5%. For patients from Group SC, a subconjunctival bleb of anesthesia was created by injecting 0.4 ml of lidocaine 1% into the subconjunctival space posteriorly to the superotemporal limbus with a 30-gauge, 1/2-inch needle attached to a 1-ml syringe. Five min after the drop of povidone iodine 5%, a sterile field and a lid speculum were placed on the eye. The injection site was measured with calipers to be 3.5 mm or 4.0 mm posterior to the superotemporal limbus, for pseudophakic and phakic eyes, respectively. A 30-gauge 1/2-inch needle was used to inject 0.05 ml of bevacizumab (Avastin; Genentech, Inc.). After the injection, mild pressure was applied with a swabstick over the injection site to reduce vitreous reflux and subconjunctival hemorrhage and another drop of povidone iodine 5% was instilled.
Immediately following the injection, a nurse who was masked to the treatment, explained the 100-mm visual analog scale for pain (Figure 1) and recorded the level of pain perceived by patients during the injection. This assessment was repeated 10 min, 1 h, 6 h, and 24 h later, without visualization of prior responses. Patients were also asked to grade their overall experience with the injection procedure as (5) Excellent, (4) Very Good, (3) Fair, (2) Poor, or (1) Awful. The physician rated patients’ eye movements during the intravitreal injection on 3 levels: (0) none or minimal, (1) not compromising the injection, or (2) compromising the injection. Complications that occurred during or after the procedures were also recorded.
Statistical analysis
Demographic, clinical, and pain characteristics of patients were analyzed descriptively. For categorical variables, absolute and relative frequencies were presented and for variables of a numerical nature (age and pain), summary measures (mean, standard deviation, range, quartiles, minimum, and maximum) were presented. Quartiles, minimum, and maximum values were also represented using box-plot diagrams.
Distributions of sex and clinical variables of a categorical nature were compared between groups using the chi-square test, or alternatively in the case of small samples, the Fisher exact test. When differences were detected, the standardized adjusted residuals were used to identify local differences. Data with absolute values >1.96 indicate evidence of a local difference between the categories for these cells.
The mean ages of patients receiving the treatments were compared using the analysis of variance (ANOVA) considering that at least 1 of the normality assumptions was verified employing the Kolmogorov-Smirnov test.
Because of a lack of normality, evaluation of the variable pain was not possible using ANOVA. Therefore, to assess pain behavior over time, the non-parametric Friedman test was used. Comparison of pain between treatments at each time point was performed using the Kruskal-Wallis test. If differences were detected in the Friedman or Kruskal-Wallis tests, the multiple comparison Bonferroni-Dunn test was used to reveal such differences, while maintaining the overall level of significance.
A significance level of 5% was used for all statistical tests. Statistical analyzes were performed in SPSS for Windows (SPSS for Windows Version 2.0, Chicago, IL, USA).
RESULTS
A total of 92 patients were included in the study and allocated to 1 of the 3 groups: Group Drops (n=31; 33.7%); Group SC (n=30; 32.6%); and Group Gel (n=31; 33.7%). The mean age was 66.4 years (SD=11.6, range 43-91), with no statistically significant differences between groups (p=0.434). We found no differences for sex, ocular disease, presence of systemic arterial hypertension, diabetes mellitus, or the use of anticoagulants. Demographic data is shown in table 1. There were significant differences in the degree of kinesis (p<0.001) among treatment groups. Group Drops had the highest occurrence of movements compromising intravitreal injections (38.7%), compared with a percentage of just over 3% for Group SC and Group Gel. Group SC had the highest percentage of minimal or no eye movement (83.3%).
Table 1 Demographic characteristics of patients who underwent intravitreal injections under 3 different anesthetic approaches
| Anesthetic approach | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Drops | Group SC | Group Gel | Total | |||||||||
| N | % | N | % | N | % | N | % | p value* | ||||
| Sex | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.715 | |||
| Female | 17 | 54.8% | 14 | 46.7% | 14 | 45.2% | 45 | 48.9% | ||||
| Male | 14 | 45.2% | 16 | 53.3% | 17 | 54.8% | 47 | 51.1% | ||||
| Disease | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.103a | |||
| Age-related macular degeneration | 9 | 29.0% | 11 | 36.7% | 11 | 35.5% | 31 | 33.7% | ||||
| Diabetic macular edema | 22 | 71.0% | 13 | 43.3% | 15 | 48.4% | 50 | 54.3% | ||||
| Branch retinal vein occlusion | 0 | 0.0% | 5 | 16.7% | 4 | 12.9% | 9 | 9.8% | ||||
| Central retinal vein occlusion | 0 | 0.0% | 1 | 3.3% | 1 | 3.2% | 2 | 2.2% | ||||
| Sistemic arterial hypertension | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.409 | |||
| No | 14 | 45.2% | 9 | 30.0% | 10 | 32.3% | 33 | 35.9% | ||||
| Yes | 17 | 54.8% | 21 | 70.0% | 21 | 67.7% | 59 | 64.1% | ||||
| Diabetes mellitus | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.349 | |||
| No | 12 | 38.7% | 17 | 56.7% | 16 | 51.6% | 45 | 48.9% | ||||
| Yes | 19 | 61.3% | 13 | 43.3% | 15 | 48.4% | 47 | 51.1% | ||||
| Anticoagulant use | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.417a | |||
| No | 29 | 93.5% | 25 | 83.3% | 29 | 93.5% | 83 | 90.2% | ||||
| Yes | 2 | 6.5% | 5 | 16.7% | 2 | 6.5% | 9 | 9.8% | ||||
| Aspirin use | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.147 | |||
| No | 26 | 83.9% | 27 | 90.0% | 22 | 71.0% | 75 | 81.5% | ||||
| Yes | 5 | 16.1% | 3 | 10.0% | 9 | 29.0% | 17 | 18.5% | ||||
*= p value (chi-square test or Fisher's exact test(a));
Group Drops= proparacaine 0.5% drops; Group SC= proparacaine 0.5% drops plus subconjunctival lidocaine; Group Gel= 2% lidocaine gel.
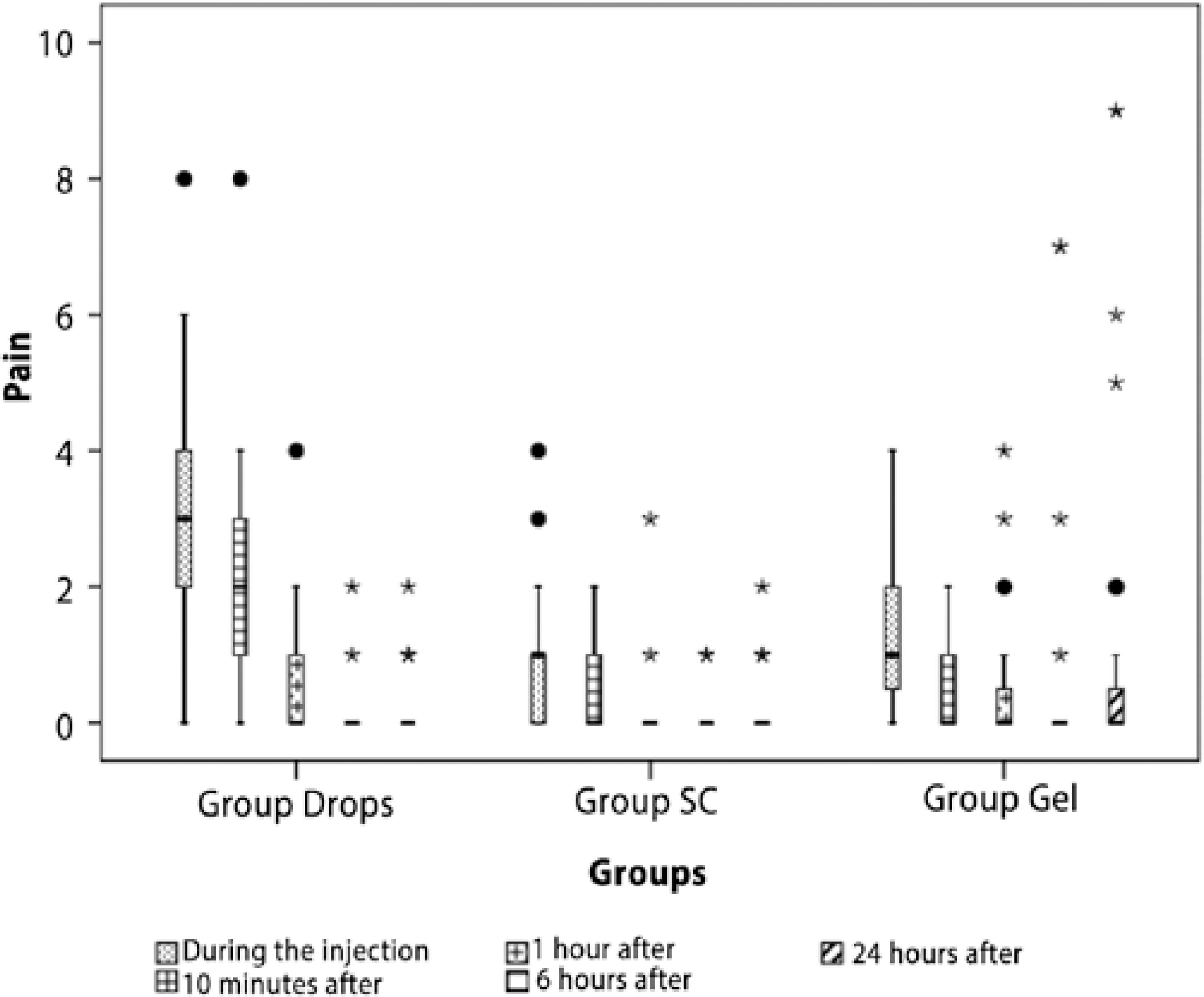
Pain scores over time in the 3 treatment groups are presented in figure 2. During the injection and 10 min after the injection, the Group Drops patients suffered higher levels of pain compared with the other 2 groups, which were similar to each other. At 1 h after the injection, there was a significant difference in the level of pain between Group Drops and Group SC but not between Group Gel and the other groups. Six and 24 h after surgery, there was no statistically significant difference in pain levels among the 3 groups.
There was higher frequency of Poor (19.4%) and Fair (61.3%) experiences reported in Groups Drops compared with the other 2 groups. Conversely, Group SC had the highest frequency of Excellent experiences (37.9%), and Group Gel had the highest percentage of Very Good experiences (67.7%; Table 2).
Table 2 Overall experience during intravitreal injections under 3 different anesthetic approaches
| Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Drops | Group SC | Group Gel | Total | |||||||||
| N | % | N | % | N | % | N | % | p value* | ||||
| Experiment procedure as a whole | 31 | 1000% | 30 | 100.0% | 31 | 100.0% | 91 | 100.0% | <0.001a | |||
| Awful | 0 | 0.0% | 0 | 0.0% | 3 | 9.7% | 3 | 3.3% | ||||
| Poor | 6 | 19.4% | 0 | 0.0% | 1 | 3.2% | 07 | 7.7% | ||||
| Fair | 19 | 61.3% | 4 | 13.8% | 5 | 16.1% | 28 | 30.8% | ||||
| Very good | 6 | 19.4% | 14 | 48.3% | 21 | 67.7% | 41 | 045.1% | ||||
| Excellent | 0 | 0.0% | 11 | 37.9% | 1 | 3.2% | 12 | 13.2% | ||||
*= p value (chi-square test or Fisher's exact test(a));
Group Drops= proparacaine 0.5% drops; Group SC= proparacaine 0.5% drops plus subconjunctival lidocaine; Group Gel= 2% lidocaine gel.
The ophthalmologist detected significant differences in eye movement during the injection among treatment groups (p<0.001). Group Drops had the highest occurrence of movements compromising the injection (38.7%), compared with just over 3% in the other 2 groups. Group SC had the highest frequency of minimal or no movement (83.3%; Table 3).
Table 3 Ophthalmologist's perception of eye movement during intravitreal injections under 3 different anesthetic approaches
| Anesthetic approach | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Drops | Group SC | Group Gel | Total | |||||||||
| N | % | N | % | N | % | N | % | p value* | ||||
| Degree of eye movement | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | <0.001a | |||
| Minimum or absent | 4 | 12.9% | 25 | 83.3% | 16 | 51.6% | 45 | 48.9% | ||||
| Not compromising the injection | 15 | 48.4% | 4 | 13.3% | 14 | 45.2% | 33 | 35.9% | ||||
| Compromising the injection | 12 | 38.7% | 1 | 3.3% | 1 | 3.2% | 14 | 15.2% | ||||
*= p value (chi-square test or Fisher's exact test(a)).
Group Drops= proparacaine 0.5% drops; Group SC= proparacaine 0.5% drops plus subconjunctival lidocaine; Group Gel= 2% lidocaine gel.
As shown in table 4, there were significant differences among the groups for the presence of chemosis (p=0.017) and keratitis (p=0.003). Group SC had the highest occurrence of chemosis (16.7%). Group Gel had the highest incidence of keratitis (19.4%). No differences in postoperative hyperemia or hyposphagma were detected. There were no cases of infection or lens damage.
Table 4 Complications of intravitreal injections under 3 different anesthetic approaches
| Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group Drops | Group SC | Group Gel | Total | |||||||||
| N | % | N | % | N | % | N | % | P* | ||||
| Hyperemia | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.326 | |||
| No | 18 | 58.1% | 13 | 43.3% | 19 | 61.3% | 50 | 54.3% | ||||
| Yes | 13 | 41.9% | 17 | 56.7% | 12 | 038.7% | 42 | 045.7% | ||||
| Hyposphagma | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.190 | |||
| No | 22 | 71.0% | 15 | 50.09% | 21 | 67.7% | 58 | 63.0% | ||||
| Yes | 9 | 29.0% | 15 | 050.0% | 10 | 032.3% | 34 | 037.0% | ||||
| Chemosis | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.017a | |||
| No | 30 | 96.8% | 25 | 83.3% | 31 | 100.0% | 86 | 93.5% | ||||
| Yes | 1 | 3.2% | 5 | 016.7% | 0 | 0.0% | 6 | 006.5% | ||||
| Keratitis | 31 | 100.0% | 30 | 100.0% | 31 | 100.0% | 92 | 100.0% | 0.003a | |||
| No | 31 | 100.0% | 30 | 100.0% | 25 | 80.6% | 86 | 093.5% | ||||
| Yes | 0 | 0.0% | 0 | 0.0% | 6 | 19.4% | 6 | 6.5% | ||||
*p= value (chi-square test or Fisher's exact test(a)).
Group Drops= proparacaine 0.5% drops; Group SC= proparacaine 0.5% drops plus subconjunctival lidocaine; Group Gel= 2% lidocaine gel.
There was no statistically significant correlation between the use of aspirin or other anticoagulant drugs and the occurrence of hyperemia or hyposphagma (Table 5).
Table 5 Use of aspirin or other anticoagulant drugs and incidence of postoperative hyposphagma
| Hyposphagma | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | |||||||
| N | % | N | % | N | % | p value* | |||
| Aspirin | 58 | 100.0% | 34 | 100.0% | 92 | 100.0% | 0.339 | ||
| No | 49 | 84.5% | 26 | 76.5% | 75 | 81.5% | |||
| Yes | 9 | 15.5% | 8 | 23.5% | 17 | 18.5% | |||
| Anticoagulant | 58 | 100.0% | 34 | 100.0% | 92 | 100.0% | 0.721a | ||
| No | 53 | 91.4% | 30 | 88.2% | 83 | 90.2% | |||
| Yes | 5 | 8.6% | 4 | 11.8% | 9 | 9.8% | |||
*= p value (chi-square test or Fisher's exact test(a).
DISCUSSION
Intravitreal injections are one of the most widely performed ophthalmic procedures, but there is no consensus regarding the best method of topical anesthesia. Intravitreal injections may be required as frequently as every month. Therefore, is important to minimize pain and complications and to maximize patient comfort. Many topical anesthetics are available but the most commonly used in our practice are proparacaine drops, subconjunctival lidocaine, and lidocaine gel. To our knowledge, this is the first study comparing these 3 anesthetics for intravitreal injections.
Proparacaine drops are easy to administer, inexpensive, and have minimal side effects( 8 ). A previous study found that topical proparacaine drops, compared to 4% lidocaine solution or 3.5% lidocaine gel, provided a very effective and cost-effective anesthesia during office-based intravitreal injections( 9 ). One study comparing proparacaine drops with tetracaine, lidocaine pledgets, and subconjunctival injections of lidocaine for intravitreal injections, concluded that proparacaine drops had the lowest average combined pain score( 10 ). Another study comparing topical proparacaine drops, xylocaine sub-conjunctival injections, and xylocaine peribulbar injections before intravitreal injections, showed no significant difference in pain scores between drops and subconjunctival injections for injection-related and entire procedure pain scores( 5 ).
In the present study, the patients treated with proparacaine drops had more troublesome eye movements during intravitreal injections (38.7 % compromising the injection) and higher pain scores during and 10 min after the procedure compared with the other 2 groups. The patients treated with proparacaine drops also reported the worst overall experience during the procedure, with 61.3% and 19.4% rating it Fair or Poor, respectively. However, the pain scores after 1, 6, and 24 h were similar to those in the other groups, and there were low incidences of hyposphagma and chemosis.
A previous study compared lidocaine 4% absorbed by a surgical sponge and subconjunctival injection of lidocaine 4% for anesthesia before intravitreal injections. Despite a significantly lower pain score during the injection in the subconjunctival group, its application was more painful and the overall procedure pain scores were similar( 6 ).
In the present study, the patients who underwent a subconjunctival injection of lidocaine 2% before intravitreal injections had less troublesome eye movement (83.3% rated as minimum or absent) and reported a better experience during the procedure, with 37.9% and 48.3% rating it Excellent or Very Good, respectively. However, these patients suffered the highest incidence of chemosis (16.7%) among the 3 groups. The incidence of hyperemia or hyposphagma was also high in the group (50%) which underwent a subconjunctival injection of lidocaine, but the difference among the groups was not statistically significant. In previous studies, the main complication after subconjunctival anesthesia is hyposphagma, occurring in up to 56% of patients( 6 , 11 ). The lack of a significant result in the present study may be a result of the sample size and the high incidence of hyposphagma in Group Drops (29%) and Group Gel (32.3%).
Lidocaine gel is commonly used for topical anesthesia because it has the advantage of increased contact time with the ocular surface and sustained lidocaine release( 12 ). Several studies have compared the anesthetic effect of lidocaine gel and subconjunctival lidocaine for intravitreal injections. Friedman et al.( 11 ) identified a trend to less pain in the gel group, but the difference was not statistically significant. Kozak et al.( 10 )found no differences in patient comfort or ease of application between the 2 groups.
In the present study, the patients treated with 2% lidocaine gel showed minimal to moderate eye movements (51.6% minimum or absent and 45.2% moderate eye movement, but without disturbing the injection). These patients also reported a good experience during the procedure, with 67.7% rating the experience as Very Good. Pain during and after the injection was generally similar to Group SC and better than Group Drops but there was a trend to higher pain scores after 24 h. We attribute this finding to the higher incidence of keratitis in Group Gel (19.4%). All cases of keratitis were successfully treated with lubricant eye drops. There were no cases of chemosis in Group Gel.
Despite the reported anesthetic efficacy of lidocaine gel there is concern about a possible increased risk of post-procedure infection. Lidocaine gel could block the contact of povidone iodine with bacteria on the eye, so it should be applied after the povidone iodine( 13 ). Despite this concern, Inman et al.( 14 )reported no cases of endophthalmitis after 4690 intravitreal injections using 2% lidocaine gel for anesthesia. There were no cases of endophthalmitis in any group in the present study.
The use of aspirin and anticoagulants is very frequent, especially in the elderly population. In the present study, 18.5% of patients were using aspirin and 9.8% were using another anticoagulant drug. The risk of hyposphagma after the intravitreal injection was not increased in these patients (p>0.05). These results support previous studies such as the MARINA trial, which found no intraocular bleeding during 1318 consecutive injections in a total of 60 warfarin-treated participants who received a mean of 22 injections.
CONCLUSIONS
Subconjunctival lidocaine was more effective than proparacaine drops or 2% lidocaine gel in preventing pain and eye movement during intravitreal injections. Although 2% lidocaine gel provided a good overall experience for the patients, the incidence of keratitis was significant (19.4%). Therefore, we do not recommend 2% lidocaine gel as the first anesthetic choice for intravitreal injections. Despite the very low incidence of complications following the use of proparacaine drops, we do not recommend it as a single agent for anesthesia in intravitreal injections. There is no evidence to suspend the use of aspirin or other anticoagulants drugs prior to intravitreal injections.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

