INTRODUCTION
Interferons (IFNs) are a class of cytokine, some of which have the ability to inhibit cell proliferation and modulate viral replication functions of the immune system. They have wide clinical uses in multiple viral and tumorigenic processes, and have been classically used in the treatment of severe diseases such as hepatitis C and multiple sclerosis. IFN is also the only accepted adjuvant treatment for patients with cutaneous melanoma(1). Although generally well tolerated, incidences of IFN toxicity are commonly reported. The most common side effects are fever, myalgia, arthralgia, and headache. Retinal damage may also be associated with its use(2,3). Furthermore, more serious disorders such as neurologic alterations, hepatotoxicity, and hematopoietic or cardiovascular irregularities, have been associated with IFN treatment(2,3).
While IFN-associated retinopathy appears in 18-86% of patients treated with the drug, (especially in patients suffering from hepatitis C, for whom IFN is widely administered), many of these cases are asymptomatic(4). In a previous report describing patients with resected high-risk melanoma who received adjuvant IFN, 13% developed retinopathy(5).
Since most of the adverse effects are due to toxicity, these symptoms usually resolve or improve after therapy discontinuation(1). Furthermore, there is a direct relationship between the dose and duration of treatment and the likelihood of retinopathy, with its appearance occurring between 8 and 12 weeks after the beginning of treatment(6).
However, in some cases complications that result in significant visual impairment can occur. Thus, monitoring and periodic ophthalmologic evaluation of patients undergoing IFN treatment is highly recommended(1,2). Here we report a case of atypical IFN-associated retinopathy involving macular changes, appearing in a young woman with cutaneous melanoma.
CASE REPORT
A 35 year-old woman presented with a history of spreading malignant melanoma of 1.1 mm Breslow thickness on her left arm. The melanoma was diagnosed in 2005. Four months ago, in a routine PET/CT extension study, metastases with bilateral axillary extensions were observed in both breasts. This required radical surgery, axillary lymphadenectomy, and radiotherapy. Oncogene mutation testing of BRAF V 600 E was positive. The patient was diagnosed with stage IV melanoma and was treated with IFN alpha-2b.
The patient received induction treatment of 33 million units (20 million units∕m²) of IFN alpha-2b intravenously, five days per week for four weeks. Subsequent maintenance therapy of 16 million units (10 million units∕m²) subcutaneously, three days per week, was initiated for a planned total of 11 months.
After three months of treatment, the patient came to our emergency room reporting visual loss and proptosis in the left eye for the previous two weeks, along with nausea and vomiting. The patients' ophthalmologic background comprised a strabismus surgery in childhood and later amblyopia of the right eye.
In the ensuing ophthalmologic examination, VA was 0.1 in the right eye and 0.4 in the left eye. A residual esotropia of 10º in the right eye and a moderate proptosis in the left eye was detected, with extraocular and intraocular movements intact. The anterior segment and intraocular pressure appeared normal.
Fundus examination revealed multiple cotton wool spots along the vascular arcades and at the mid-peripheries of both eyes, in addition to peripapillary exudative lesions and mild venous tortuosity (Figure 1).
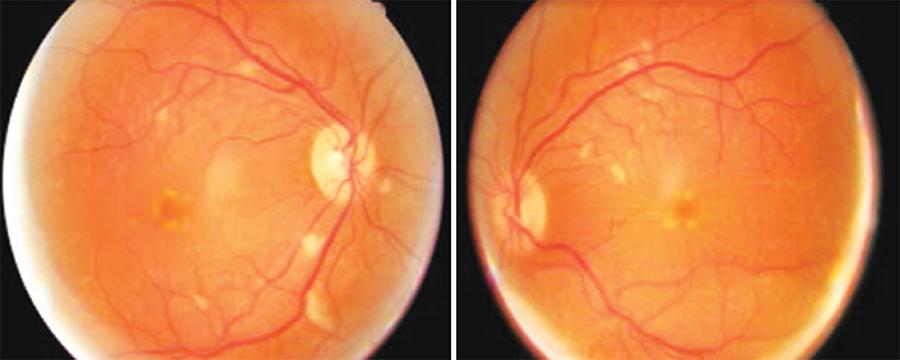
Figure 1 Fundus exam of the patient upon arrival in the emergency room following the use of interferon for three months. Cotton wool spots and peripapillary along the vascular arcades in both eyes can be observed.
Fluorescein angiography (FA) was performed, which showed lack of perfusion areas by capillary closure coinciding with the cotton wool spots and pigmentary changes surrounding both foveas. An optical coherence tomography (OCT) examination of the macula revealed the presence of hard exudates in the outer plexiform layer of both eyes, and an epiretinal membrane (EM) in the left eye, possibly associated with exudative retinal microangiopathy secondary to the use of IFN (Figures 2 and 3).
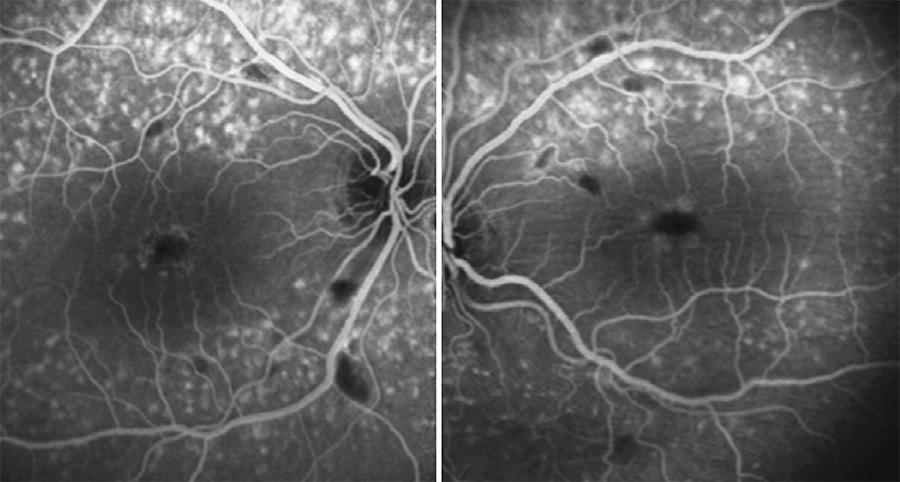
Figure 2 FA of the patient, where a lack of perfusion areas by capillary closure coinciding with cotton wool spots are shown.
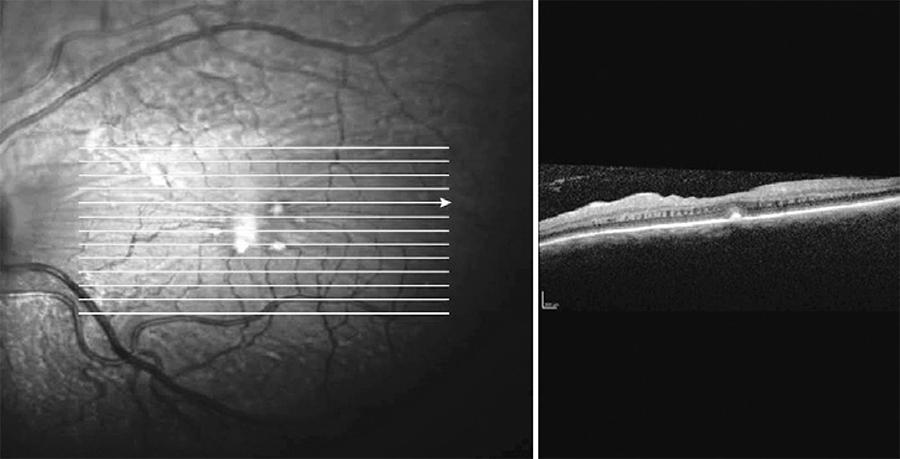
Figure 3 Macular OCT of the left eye, where hard exudates and an epiretinal membrane are observed, possibly related with the exudative retinal microangiopathy.
It should be noted that our patient had no history of diabetes mellitus, arterial hypertension, or previous cardiovascular risk factors, all of which are known to be associated with the development and progression of retinopathy in patients undergoing IFN treatment(1,7,8).
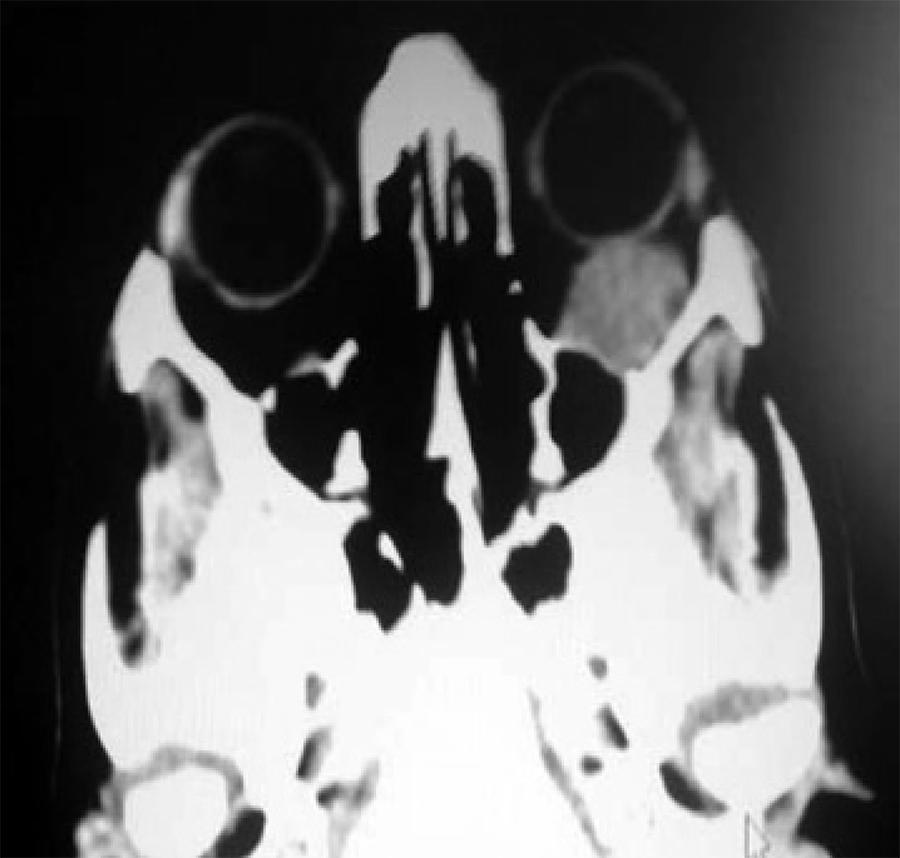
Nevertheless, given the patients' background, cerebral and orbital TC was requested, which revealed a retro-orbital mass of 15 × 25 mm compatible with orbital metastases. The mass contacted the orbit floor, without producing erosion or hyperostosis. No brain disruptions were found (Figure 4).
Additional oncological examinations, including PET/CT, revealed new chest metastases. Given the retinal involvement and the advanced tumor progression, IFN treatment was stopped. The patient was included in an experimental clinical trial of Vemurafenib, a recently described drug that blocks the production of the protein kinase BRAF. At this point the patient discontinued her ophthalmologic examinations.
DISCUSSION
Although the pathogenesis of IFN-associated retinopathy remains unknown, it is believed to be caused by immune complex aggregations in ocular vessel walls. This is thought to increase leukocyte adhesion, which may trigger microcirculation obstruction(1-3). Furthermore, it has been reported that IFN induces thrombogenic antibody proliferation(9). Typical ocular complications associated with its use include ischemic retinopathy associated with cotton wool spots, retinal hemorrhages, and peripheral vascular obstructions(7,9). Microaneurysms, neovascular glaucomas, and ischemic optic neuropathies have also been observed(2,7,8).
Currently, IFN is the only accepted adjuvant treatment for patients with high-risk relapse melanoma(1). We describe here a case of IFN-associated retinopathy in a patient with metastatic melanoma. This was diagnosed from several lines of evidence. Firstly, a fundus examination revealed the typical cotton wool spots associated with retinopathy. Also, symptoms appeared within three months following IFN intake, and the patient presented no comorbidities such as diabetes or hypertension, which could mask or aggravate the ocular findings. The bilateral nature of the findings are also suggestive of IFN-associated retinopathy, although the patient only realized visual loss in her dominant eye.
However, we suggest that a differential diagnosis should be considered for melanoma associated retinopathy (MAR) and radiation retinopathy. MAR is a rare autoimmune retinopathy that usually presents as night blindness and mild peripheral vision loss, with acuity and colour vision preserved. Substantial differences could differentiate both processes, since MAR is observed mainly in male patients and is usually not reversible with treatment discontinuation(1,10). Radiation retinopathy might also be considered in the diagnosis, since it often shows clinical similarities. These include the vascular compromise on the posterior retina, which is normally more sensitive to radiation than the peripheral retina. Nevertheless, radiotherapy in our patient was administered long before IFN therapy and visual impairment was not previously reported(11).
It is possible that after discontinuation of IFN therapy most of the retinal lesions would have spontaneously disappeared, given the reversibility of IFN-associated side effects previously reported in multiple studies(1,2). However, the prognosis of our patient was overshadowed due to the advanced level of systemic tumor progression, that made it impossible to attend follow-up visits.
This case was also unusual because we detected some retinal changes not always observed in this kind of retinopathy. In particular, perimacular exudative damage that might be due to a transient period of choroidal hypoperfusion, appeared to have caused changes in the RPE. This may have formed an EM and thus resulted in long-term visual impairment. Choroidal vascular occlusion has also been described in patients under IFN therapy, but this usually occurs with associated comorbidities such as diabetes mellitus or hypertension(8,9).
We note that the atypical features presented here occurred in a patient with no history of notable coexisting pathologies, and emphasise that in such cases vision-threatening complications might still occur. Therefore, it may be difficult to weigh the risk of continued IFN treatment against its undoubted benefits(8). We therefore recommend that before initiating treatment, patients undergoing IFN therapy, should be informed of its potential ocular risks and, more importantly, they should be subjected to periodic ophthalmologic examinations.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

