INTRODUCTION
The hepatitis C virus infection is a severe disease that can become chronic and progress to hepatic decompensation, cirrhosis, and in 20% of the cases to hepatocellular carcinoma(1). Around 3% of the world's population (170 million people) is estimated to be the chronic carrier of the hepatitis C virus(2). In Brazil, prevalence of this disease ranges from 0.9% to 1.9%, proportionate to the population of each state(3).
The current FDA approved and recommended treatment for hepatitis C is the interpheron-alpha monotherapy, which includes pegylated interferon α-2b or along with ribavirin. Interferon is an immunomodulatory compound with direct antiviral and antiproliferative properties; it has been used as a therapeutic for hepatitis C, B, and D, cancer, and multiple sclerosis. In vitro, interferon alpha inhibits viral replication(4). Ribavirin is an immunomodulatory compound that inhibits viral DNA and RNA. When used alone it has no affect on hepatitis C viral replication(5). However, when combined with interferon, viral and biochemical response is sustained, when compared to the stand-alone use of interferon alpha(6). The treatment duration of 24 to 48 weeks has been recommended(6).
The use of interferon as the treatment for hepatitis C in patients might lead to systemic changes, such as myalgia, fever, erythema, weakness, loss of appetite, anemia, and thrombocytopenia(1). Ribavirin has a fewer side effects among which hemolytic anemia being the most frequent one(7). Studies have reported an association of these compounds with optic neuropathy(7), retinopathy(8-14), subconjunctival hemorrhage(8), cystoid macular edema(15), and retinal and choroidal perfusion deficiency(16).
The objective of this study is to identify possible alterations in ocular fundus examination upon start of the treatment, as well as to analyze the changes in visual acuity and visual field.
METHODS
This was a prospective observational study performed at the Hepatology Clinic of the São José Regional Hospital and at the Vitreoretinal Department at the Sadalla Amin Ghanem Eye Hospital. The study was conducted in the patients with chronic hepatitis C immediately commencing a standard 48-week treatment regimen of pegylated interferon α-2b (1.5 mcg/kg/week) subcutaneous injections and ribavirin twice daily dosage of 800 mg. The study was approved by the Ethics Board under number 196824. All patients signed the informed consent form.
All the patients were interviewed regarding systemic diseases and were evaluated with the following ophthalmologic evaluation items before starting the treatment, after one month and quarterly up to twelve months. Examination consisted of best corrected visual acuity (subjective refraction); biomicroscopy; intraocular pressure (Goldmann applanation tonometry); retinal mapping with indirect ophthalmoscope (using a 20D lens); direct and consensual pupillary light reflex test; visual field analysis with automated perimetry (Humphrey Field Analyzer II 750i, HFA 750i), with 24-2 SITA-standard strategy (Swedish Interactive Threshold Algorithm). Patients with concomitant diabetes mellitus underwent to fluorescein angiography exam (Topcon, TRC-50IX system, IMAGEnet 2000, intravenous 2.5 ml of 20% sodium fluorescein solution) before starting the treatment, after one month, and once in three months for up to twelve months. The glycosylated hemoglobin dosage was monitored in the same periods.
Patients with the following characteristics were excluded: age below 18 years; diabetic retinopathy with changes in retinal perfusion at fluorescein angiography exam on initial evaluation or uncontrolled clinical diabetes upon follow-up (such as glycosylated hemoglobin-HbA1c over 8%) retinopathy; central or brain retinal vein occlusion; systemic arterial hypertension with retinopathy upon initial evaluation, or uncontrolled clinical systemic arterial hypertension upon follow-up during the existence of retinopathy; other type of retinal vasculopathy arising due to non-infectious (Behçet's disease, Wegener granulomatosis, systemic lupus erythematous, polyarthritis nodosa, Crohn's disease, sarcoidosis, multiple sclerosis), infectious (syphilis, Lyme's disease, toxoplasmosis, toxocariasis, tuberculosis, herpes, mononucleosis, leptospirosis), and ocular concomitant diseases (Birdshot chorioretinopathy, pars planitis, Eales disease, IRVAN syndrome, multifocal choroiditis).
RESULTS
Nineteen patients, 7 females and 12 males of 21 to 65 years of age were enrolled in the study (mean, 46 years). The duration of follow-up varied from 4 to 48 weeks. Three patients missed the follow up after first examination and were excluded. Out of sixteen patients two were followed up to one month (12.5%), three patients for 3 to 6 months (18.75%), three patients for 6 to 9 months (18.75%), and eight from 9 to 12 months (50%). One patient was followed for 24 months. Average follow-up time was 8.18 months.
Retinopathy developed in six patients (37.5%) and among these two patients (12.5%) showed unilateral retinal hemorrhage presenting with a small and isolated hemorrhage; cotton-wool spots characterized by small, isolated whitish spots, not associated with changes in visual function (Figure 1) were observed in four patients (25%) or six eyes (18.75%).
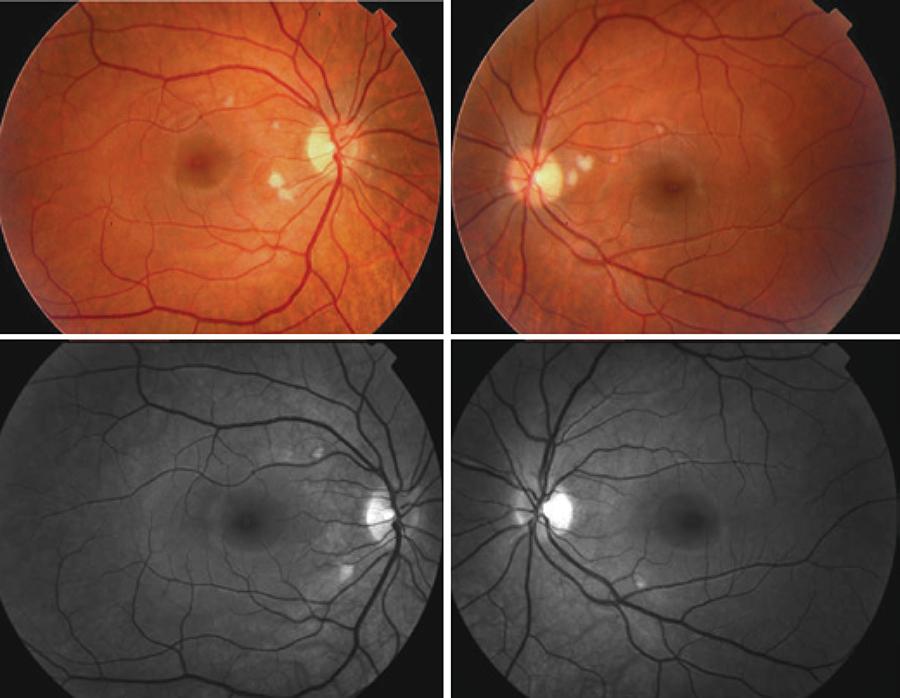
Figure 1 Color (upper) and red-free retinograph (below) of both eyes of patient 1 at 6-month follow-up treatment for hepatitis C, presenting with multiple cotton-wool spots without associated hemorrhage, smaller in the superior papillomacular bundle area and bigger in the inferior one.
Out of 16 patients, a group of patients were diagnosed with the following systemic diseases: HIV, 1 (6.25%); diabetes, 3 (18.75%); 3 patients had known treated hypertension (18.75%); and liver failure, 1 (6.25%). After the beginning of treatment, 66.6% of the patients developed diabetic retinopathy and the same occurred with hypertensive patients. Of the total of detected retinopathy, hypertension accounted for 33.3%. The same was true for patients with diabetes.
These patients were instructed to continue systemic treatment with an ophthalmologic evaluation every two months and return if any ophthalmologic signal was detected. All patients adequately completed the treatment without any interruption due to the severity of ophthalmic associated findings.
Upon completion of the treatment and terminating the medication the patients showed no signs of retinopathy or visual damage (Figure 2).
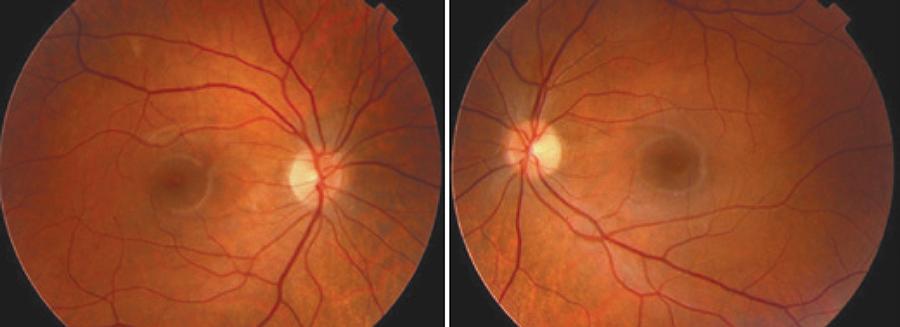
Figure 2 Color retinography of both eyes of patient 1 showing no retinal lesions at one year follow-up.
One of the patient, patient number 6 presented bilateral decrease of visual acuity during the treatment (6.25% of patients), associated with cotton wool spots on the right eye. However, this condition was not connected with that pegylated interferon α-2b and ribavirin treatment as the condition persisted even in absence of the medication. The patient recovered after one year of interruption of the treatment (Table 1). In the initial tests, patient also showed bilateral nonspecific disturbances on peripheral visual field at 30-2 strategy, without disc edema and not compatible with retinal manifestation on the right eye.
Table 1 Profile of patients that underwent follow-up during the treatment for hepatitis C
| Age (years) | Associated disease | Ocular changes | Time of detection of ocular changes | BCVA* before treatment | BCVA* last query | |||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | |||||
| 1 | 21 | - | Bilateral cotton wool spots | month 6 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 62 | Hypertension | Bilateral cotton wool spots | month 3 | 1.0 | 1.0 | 1.0 | 1.0 |
| 3 | 42 | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 4 | 47 | Diabetes | Retinal hemorrhage in OS | week 3 | 1.0 | 1.0 | 1.0 | 1.0 |
| 5 | 47 | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 6 | 58 | - | Cotton wool spots in OD | month 5 | 1.0 | 1.0 | 1.0 | 1.0 |
| 7 | 44 | Diabetes and hypertension | Retinal hemorrhage in OS | month 6 | 1.0 | 0.5 | 1.0 | 0.5 |
| 8 | 33 | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 9 | 46 | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 10 | 51 | Diabetes and hypertension | - | - | 0.9 | 0.9 | 1.0 | 0.9 |
| 11 | 40 | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 12 | 44 | HIV** | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 13 | 36 | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 14 | 54 | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
| 15 | 42 | - | Left eye cotton wool spots | month 7 | 1.0 | 1.0 | 1.0 | 1.0 |
| 16 | 44 | Liver failure | - | - | 1.0 | 1.0 | 1.0 | 1.0 |
*BCVA= best corrected visual acuity;
**HIV= human immunodeficiency virus.
No color vision damage was found in any patient throughout the systemic treatment.
DISCUSSION
Physiopathology of retinopathy has not been clearly understood. Guyer et al.(11) suggested that immune-complex deposition and leucocyte infiltration in the retinal vasculature would lead to ischemic episodes. Earlier studies(17,18) have shown that the increase in plasma complement levels and plasma aggregation would facilitate capillary infarction, thus explaining the ischemic alterations of retinopathy.
Although treatment with pegylated interferon α-2b associated with ribavirin might lead to ocular side effects, the associated use seems to bring a few side effects(19). Irritability and eye discomfort are most commonly reported due to the drug-induced conjunctivitis as the drug is secreted along with tear(20).
The incidence of retinopathy related to pegylated interferon α-2b has been widely reported in the literature. In a systematic review from Raza et al.(21), the overall incidence of retinopathy during the treatment was around 27%. In a prospective study Vujosevic et al.(22) reported 30% of retinopathy in 97 patients. In another prospective study, Kim et al.(23) evaluated 32 patients (64 eyes) and 11 of them developed retinopathy (34.4%). Cotton-wool spots were found in six patients, both eyes (18.7%); retinal hemorrhages in four (12.5%), both eyes. Panetta et al.(24) reported in a retrospective study a very low incidence (3.8%) of retinopathy among 183 patients with chronic hepatitis C treated with pegylated interferon α-2b and ribavirin. However, only symptomatic patients were included in this study.
It is likely that the incidence of retinopathy could be under represented. The cotton-wool spots were transient, often asymptomatic with no visual acuity, so it is possible that they were not always detected, especially when the patients missed or were irregular with the follow-up. Most patients showed full resolution without visual sequelae.
Schulman et al.(25) showed among 42 patients (7%) treated with interferon and ribavirin, three patients had be stopped the treatment due to retinopathy (two) and disc edema (one) with low visual acuity. The patients regressed spontaneously after stopping the treatment, without sequelae.
Nagaoka et al.(26) suggested endothelial dysfunction as a cause of interferon-associated retinopathy, with increased manifestation within 2 weeks of treatment initiation. Schulman et al.(25) reported a 67% chance of development of retinopathy among hypertensive patients. Similar to the findings of Vujosevic et al.(22) that reported a frequency of 68% Kim et al.(23) also claimed hypertension to be a risk factor for retinopathy.
CONCLUSION
In our study, we diagnosed retinopathy in a considerable number of cases, but most patients showed no changes in visual acuity and quality. Only one patient showed a transient visual loss without loss of term impairing of the visual function. We conclude that according to our study and in agreement with studies published in literature, the treatment for hepatitis C with pegylated interferon α-2b associated with ribavirin although can cause retinal changes, usually do not lead to damages to visual function, and has a transitional character with complete anatomical and functional recovery. Although ocular involvement is rare, follow-up with an ophthalmologist is recommended throughout the course of the medication, especially if symptoms are detected.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

