INTRODUCTION
The continuous circular capsulotomy (CCC) of the anterior lens capsule is a critical step in the modern cataract surgery performed by phacoemulsification, which offers advantage for the implantation of intraocular lens correctly into the capsular bag(1). Good visualization of the anterior capsule flap is needed when performing the CCC, particularly in the absence of a red reflex(2-4). Since the lack of a red reflex results in technical difficulties(2-4), a correct CCC is useful when a more aggressive manipulation of the capsular bag is required(3). In such clinical conditions, the improvement of the anterior capsule visualization can also be of great value for surgeons, particularly for those who are learning the surgical technique(4). Additionally, the surgical procedure of staining the anterior capsule during CCC in pediatric cataract extraction is useful since the anterior capsule of these eyes is thin and elastic(5).
Since the first use of fluorescein for improving the visualization during capsulorhexis in 1993(6), alternative techniques and a variety of dyes have been used for anterior capsule staining during cataract surgery(4). Although indocyanine green, trypan blue, sodium fluorescein, crystal violet, and gentian violet have been shown to be useful for facilitating the CCC maneuver, only the trypan blue 0.06% - Vision blueTM (Dorc, Netherlands) has been approved by the FDA for use in cataract surgery(7,8).
New dyes that enhance the visualization of ocular tissues during surgery have recently emerged(9-11). Lutein and zeaxanthin are two major components of the macular pigment and are the only carotenoids found in the macula and lens of humans. The antioxidant properties of the dyes and the ability to filter blue light facilitated their use during the CCC procedure(12,13).
It has been reported that the green 1% solution of soluble lutein and zeaxanthin-based dye combined with 0.04% trypan blue (PhacodyneTM, Kemin, USA) is a useful intraocular dye for staining the anterior capsule, enhancing the CCC procedure in human cadaveric eyes(11). Its safety profile has been evaluated by clinical, histological, and electroretinographic methods after intravitreal injections into rabbit eyes(14).
The objective of this study was to evaluate the feasibility and safety of application of a novel dye comprising 1% soluble lutein-zeaxanthin together with 0.04% trypan blue (PhacodyneTM, Kemin, USA) to improve the anterior capsulorhexis during the phacoemulsification surgery in human eyes.
METHODS
Preparation of the dye
The method of preparation described below is valid for lutein/zeaxanthin regardless of the chemical form, purity, crystallization, or degree of esterification (patent office application #61/468,838).
The initial steps of this study were the development of a water-soluble solution, characterization of the absorbance of the lutein/zeaxanthin solution using the Aquamate device (Thermo Spectronic, Cambridge, UK), and the identification of the color of the chromophoric groups. The physicochemical parameters were tested for the optimum pH, osmolarity/osmolality, and concentration. Solubility in water and polyvinyl alcohol was tested, the ultraviolet spectra were recoded, and the blends showing different colors within the visible spectrum were analyzed.
The method of preparation of the dye included dissolution, agitation, sterilization, and filtration. First, the components were dissolved in balanced saline solution (BSS) or any other solution compatible with the components of the formula that provided stability, suitable pH, and osmolarity acceptable for ocular use. Sterilization using wet heat was achieved by autoclaving at 121±C. The final dye solution contained 1% soluble lutein/zeaxanthin and 0.04% trypan blue (osmolarity, 280 mOsm; density, 1.05; pH, 7.00). Aliquots of 1 mL were prepared, stored at room temperature, and were used within 30 days.
Study design
This was a prospective, consecutive, non-randomized, interventional study of 25 eyes of 25 patients performed by 25 different surgeons. The study was registered at clinicaltrials.gov under the no NCT01627977. The study was approved by the Ethics Committee of the Federal University of São Paulo and was conducted according to the Research Guidelines of the Association of Research in Vision and Ophthalmology, adhering to the Declaration of Helsinki. All patients were informed of the benefits and risks of the surgical procedure as well as the nature and possible consequences of the new dye tested. This study was conducted after receiving informed consent from all participants.
Eyes were submitted to phacoemulsification at the Ophthalmology Department of Hospital São Paulo, Universidade Federal de São Paulo, Brazil, with PhacodyneTM (Kemin, USA) assisting the CCC technique and the hydrophilic foldable IOL implantation.
Inclusion and exclusion criteria
Patients over 50 years of age with indication for cataract surgery in one eye were included in the study. Patients with any previous ocular surgeries in the eye studied, pregnancy, glaucoma, a past or present intraocular infection, and any other ocular condition detected during the pre-operative examination that could limit or affect the postoperative results were excluded. The minimum follow-up period was 30 days.
Physical evaluation and ocular examinations
All patients underwent a full ophthalmologic examination at the start of the study and postoperatively (days 1, 7, and 30). This included evaluation of the best-corrected visual acuity (BCVA) that was measured using the Snellen Charts and converted to logMAR, measurement of the intraocular pressure (IOP) using the Goldmann tonometer, and slit-lamp evaluation. Complementary exams such as fundoscopy, pachymetry, and specular microscopy (CSO, Firenze, Italy) were also performed at the same time points. Baseline cataracts were graded according to the "Lens Opacities Classification System III" (LOCS III)(15). Anterior chamber reaction was graded according to SUN Working Group(16).
Surgical procedures
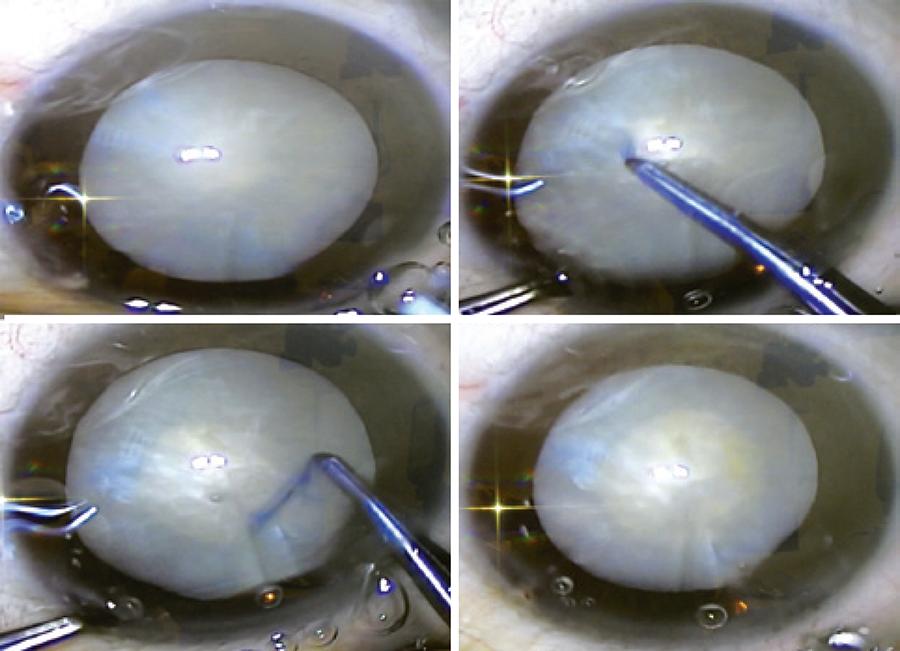
The phacoemulsification was performed by 25 different experienced cataract surgeons. The first step consisted of making a 2.75-mm clear corneal incision, filling the anterior chamber with PhacodyneTM (Kemin, USA), washing after 30 seconds with balanced salt solution, and filling the anterior chamber with viscoelastic. The anterior continuous circular capsulorhexis (CCC) (Figure 1) was performed using an Utrata forceps. This was followed by hydrodissection and hydrodelineation using approximately 0.1 mL BSS. Phacoemulsification was performed using the Infinity Vision SystemTM (Alcon, USA). Following this, the lens cortex was aspirated and a foldable intraocular lens was inserted through the 2.75-mm corneal incision (Figure 2). At the end of the surgical procedure, the viscoelastic substance was aspirated and no sutures were placed at the corneal incision. Moxifloxacyn and dexamethasone association - VigadexaTM (Alcon, USA) eye drops were used during the immediate post-operative period.
Follow-up
All eyes received 0.5% moxifloxacin eye drops 4 times daily for 7 days and 0.1% dexamethasone as part of a regressive regimen. No hypotensive eye drops were prescribed. The eyes underwent an ophthalmologic examination at days 1, 7, and 30 by expert ophthalmologists.
RESULTS
Intraoperative findings
The lutein-based dye produced a green solution (Figure 1) that stained the anterior capsule and facilitated the CCC procedure (Figure 2). The phacoemulsification followed by IOL implantation was performed as traditionally reported by cataract surgeons. No sutures were necessary.
Physical evaluation and ocular examinations at baseline and follow-up
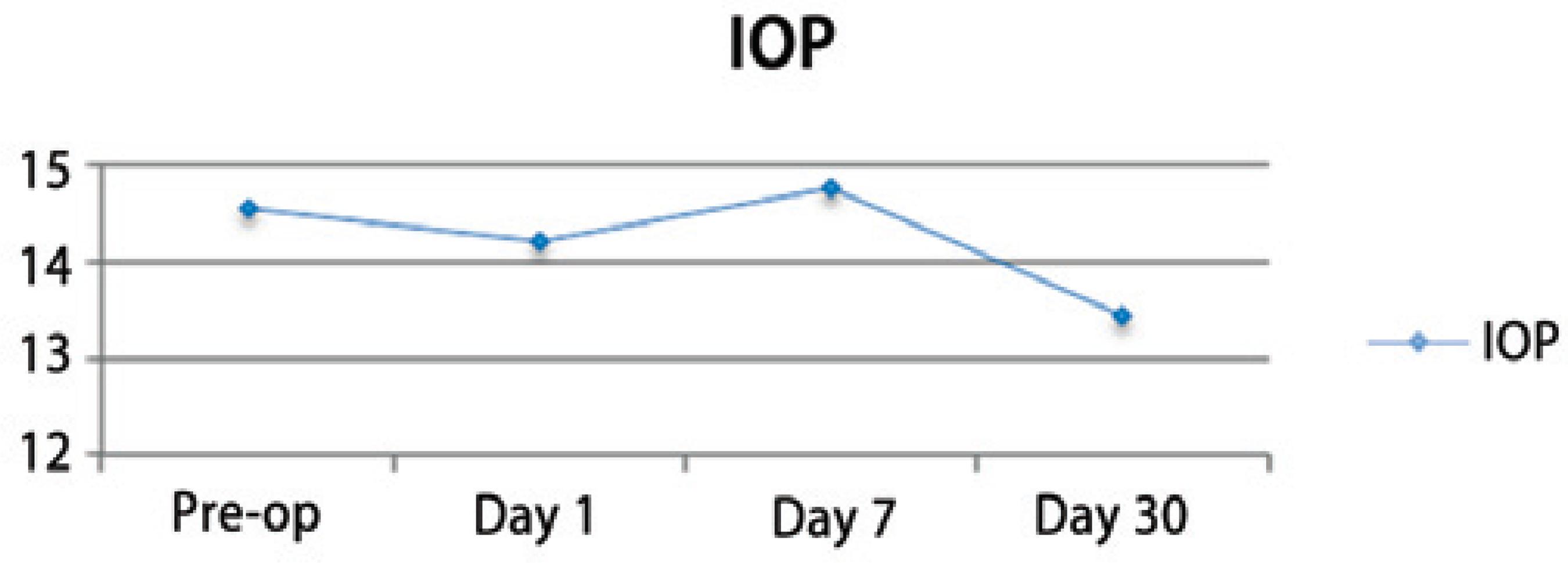
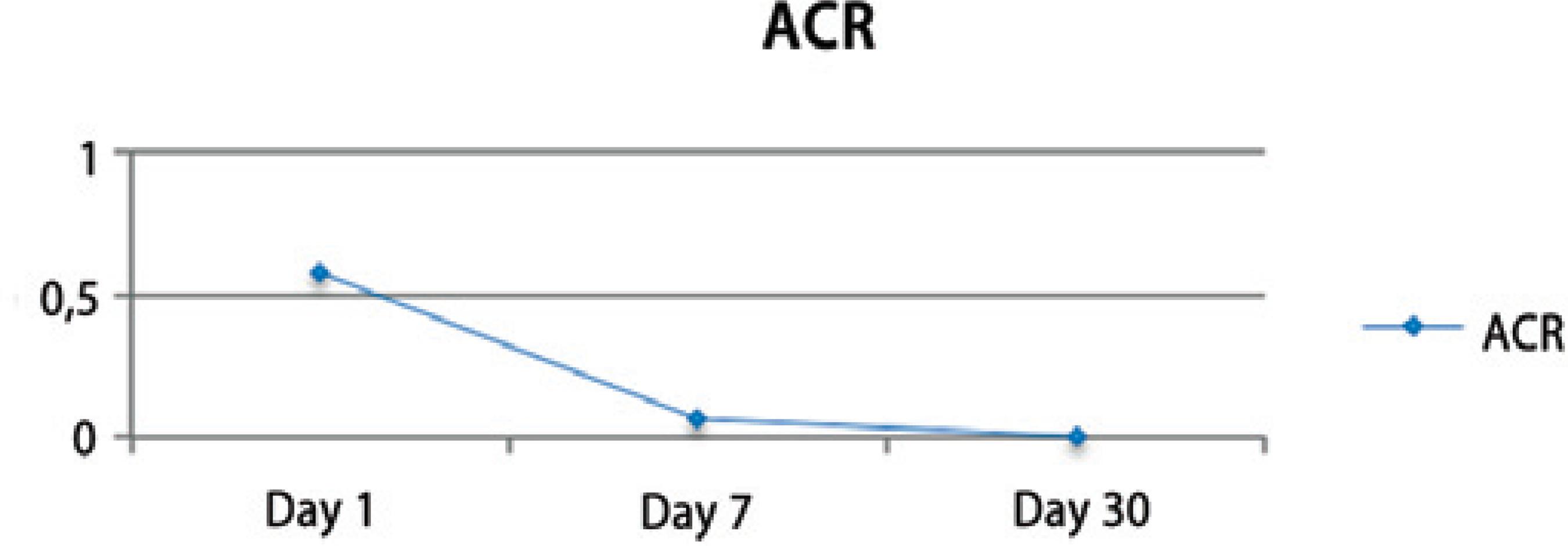
Nuclear cataract classification (according to LOCS III) was 3.24 ± 1.12 and changed from 2 to 6 in both groups. Preoperative BCVA (logMAR) was 0.89 ± 0.59 and improved to 0.23 ± 0.22 at day 30 after the surgery. The intraocular pressure (IOP) remained stable and similar to the pre-operative measurements during the post-operative visits (p=0.004) (Figure 3). A mild inflammatory reaction (grade 0.5+ according to SUN Working Group)(16) at the anterior chamber was observed within the first 7 days (Figure 4).
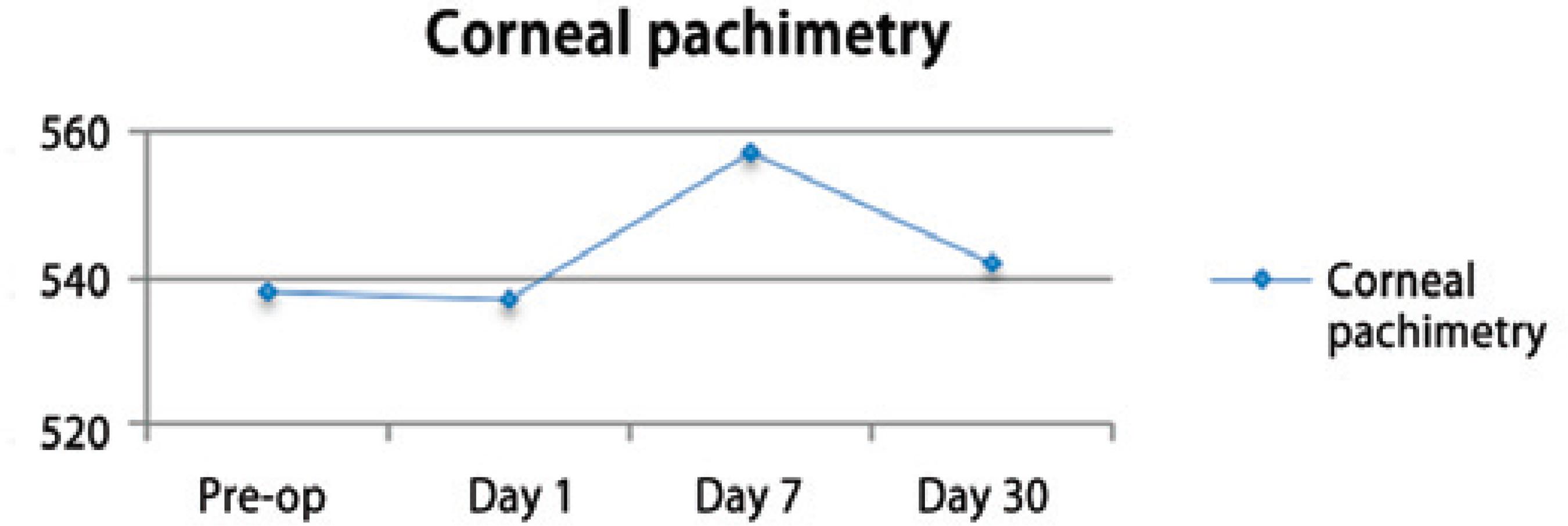
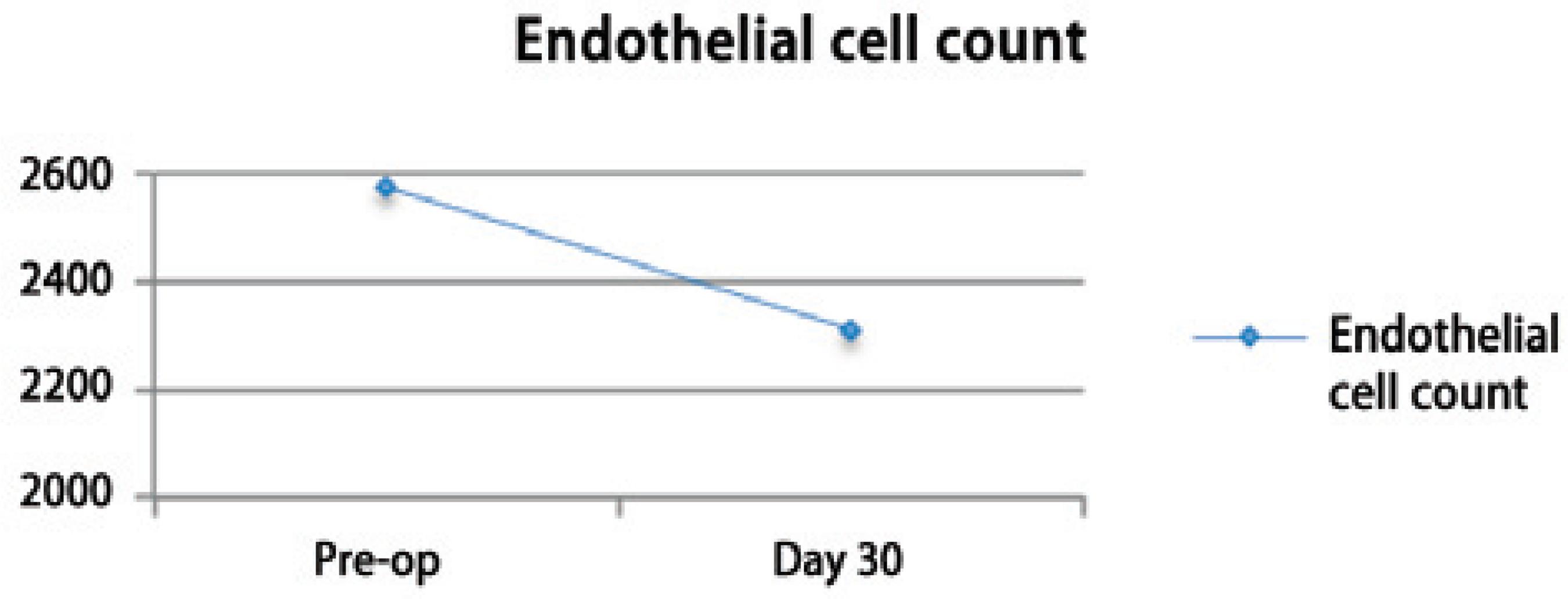
The pre-operative corneal pachymetry average was 538.8 ± 40.0 and that on post-operative follow-up day 30 was 542.5 ± 39.8 (Figure 5). The preoperative endothelial cell count average was 2573.5 ± 235.9 and that on postoperative follow-up day 30 was 2311.36 ± 490.7 (Figure 6).
There were no significant differences between the results of preand post-operative fundus biomicroscopy.
Questionnaires
The questionnaires showed that surgeons considered PhacodyneTM to be efficient for staining the anterior capsule and that the staining facilitated the CCC procedure in all eyes (Table 1).
Table 1 Questionnaire returned by surgeons showing the effectiveness of the dye
| Surgeons questionnaire response | % | p | ||
|---|---|---|---|---|
| How often do the surgeon use dyes in cataract surgery? | Never | 0 | 0% | |
| Rarely | 8 | 32% | ||
| Frequently | 13 | 52% | ||
| Always | 4 | 16% | ||
| Which dye does the surgeon use? | Trypan blue | 25 | 100% | |
| Indocyanine green | 0 | 0% | ||
| Others | 0 | 0% | ||
| How was the visualization of the anterior capsule with Phacodyne? | Good | 25 | 100% | p<0.001 |
| Bad | 0 | 0% | ||
| How was the degree of staining of the anterior lens capsule by the dye? | Nothing | 0 | 0% | p<0.001 |
| Adequate | 20 | 80% | ||
| Intense | 5 | 20% | ||
| Did it color any other ocular tissues? | No | 21 | 84% | p<0.001 |
| Yes | 4 | 16% | ||
| Conjunctiva | 0 | 0% | ||
| Iris | 1 | 4% | ||
| Posterior capsule | 0 | 0% | ||
| Incision | 2 | 8% | ||
| Cornea | 1 | 4% | ||
| What is the surgeon's opinion about the usefulness of the dye? | Good | 25 | 100% | |
| Bad | 0 | 0% | ||
| Is the color of the dye suitable for dying the anterior lens capsule? | Yes | 25 | 100% | |
| No | 0 | 0% | ||
| Were there any signs of the dye at the end of the surgery? | No | 23 | 92% | p<0.001 |
| Yes | 2 | 8% | ||
| Incision |
DISCUSSION
A previous study showed that a solution containing 1% soluble lutein-zeaxanthin and 0.04% Brilliant Blue G efficiently stained the anterior capsule and facilitated the CCC in cadaveric eyes(11). Additionally, clinical, histological, and electroretinographic evaluations performed after the intravitreal injection of the dye in rabbit eyes showed no signs of toxicity,(14) indicating the enhanced safety in case of accidental leak of the dye into the vitreous. Based on these reported we decided to evaluate the application of s the new dye for cataract surgery in humans.
Based on the nuclear cataract classification, LOCS III, the eyes enrolled in this study showed varying grades of cataract from 2 to 6, demonstrating that advanced cases of hypermature cataract were also present(6). The surgeons classified the visualization of the anterior capsule with the dye as "good" and considered it as a useful tool for cataract surgery (Table 1). The green colored dye (Figure 1) stained the anterior capsule of the lens, which facilitated the CCC procedure in all cases, including the hypermature cataracts.
The surgeons observed that in 4 eyes, the cornea, the incision, or iris were stained by the dye. Residual dye was found only in 2 eyes at the end of the surgical procedure (Table 1). The dye was not seen in any eye on the first postoperative day. A mild anterior chamber reaction of grade 0.5+ (according to SUN Working Group)(16) was observed during the first week after surgery (Figure 4). These results were similar to those of earlier clinical studies, where trypan blue was used during phacoemulsification to aid the CCC procedure or when a dye was not used.(3,4,17-19) In agreement with other reports, the IOP remained stable and similar to the pre-operative measurements during the post-operative period (Figure 3)(20).
The pre-operative BCVA (logMAR) was 0.89 ± 0.59 and improved to 0.23 ± 0.22 at day 30 after surgery, clearly showing that there was an improvement in BCVA from 20/160 to approximately 20/32 in majority of the eyes. No signs of toxicity were observed during the 30-day follow-up period(21).
Corneal pachymetric changes were observed soon after the surgical procedure and peaked at one week during the follow-up. The values returned to the baseline levels on the 30th postoperative day. The pre-operative corneal pachymetry average was 538.8 ± 40.0 and the post-operative value was 542.5 ± 39.8 (Figure 5), demonstrating that anatomical changes of the cornea were temporary and reversible within the first 30 days after the surgery(21,22).
The baseline endothelial cell count average was 2573.5 ± 235.9 and that post-operatively was 2311.36 ± 490.7 (Figure 6), showing an average decrease of 10.18% in the first month after the surgery. Similar endothelial cell loss during phacoemulsification procedures has been reported by others(21,22).
Pre-and post-operative fundus biomicroscopy evaluations showed no significant differences. Pre-and post-operative macular thickness measurements are needed to assess the effect on the retina.
To our knowledge, this is the first study that used a lutein-based dye (PhacodyneTM) for visualizing anterior capsulorhexis during cataract surgery by phacoemulsification. However, our study had limitations. This include, the small sample size, the absence of a control group, the large number of surgeons (that may affect the outcomes, although it is suitable for analyzing the efficacy), and the limited follow up period of 30 days. The goal of this study using lutein and zeaxanthin combined with trypan blue was to evaluate the safety and efficacy of the dye for CCC. Further studies are warranted to evaluate the effectiveness of the new dye and its advantages over those that are currently being used. We hypothesize that use of lutein and zeaxanthin in combination with trypan blue may have two favorable effects: 1) The lower concentration of trypan blue (that can also be tested in more reduced concentrations in future studies(23)), and 2) the antioxidant effect of lutein and zeaxanthin molecules that may quench the singlet oxygen generated by the exposure of trypan blue to light. Additionally, since lutein is a natural product, the manufacturing costs could be reduced. The use of lutein and zeaxanthin without trypan blue will be investigated in future studies.





 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

