INTRODUCTION
Keratectasia is a well-documented complication of laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK), although it is more common after LASIK(1-4). Potential risk factors include, but are not limited to, high attempted correction, thin residual corneal thickness, flap creation, irregular corneal topography, clinical keratoconus, and undiagnosed subclinical keratoconus(1).
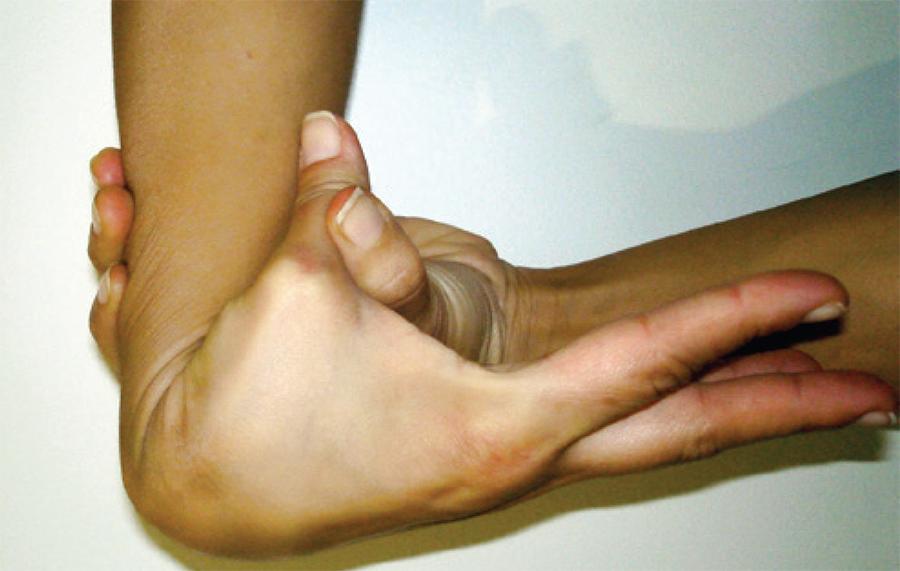
Benign joint hypermobility syndrome (BJHS) is a connective tissue disorder with hypermobility in which musculoskeletal symptoms occur in the absence of systemic rheumatological disease(5). This syndrome is considered to be an inherited connective tissue disorder(6). The primary clinical manifestations of BJHS are hypermobility and pain in multiple joints. It is different from other disorders that cause local joint hypermobility and generalized joint laxity, such as Marfan syndrome and Ehlers-Danlos syndrome (EDS).
Hypermobility that is not associated with systemic disease occurs in 4% -13% of the population(6). The hypermobility diminishes as one ages, and it also appears to be related to sex and race6. Individuals of African, Asian, and Middle Eastern descent also have increased joint laxity(7-9).
Here we describe the case of a 27-year-old woman with BJHS who keratectasia after LASIK (Figures 1, 2).
CASE REPORT
A 27-year-old woman with BJHS presented for a refractive surgery evaluation in August 2000. Her corrected distance visual acuity (CDVA) was 20/20 with a manifest refraction of -4.50 -0.75 × 180 in the right eye and 20/20 with a manifest refraction of -4.00 -1.25 × 180 in the left eye. There was no significant history of ocular trauma or a family history of keratoconus, and she had no eye rubbing habit or other ocular issues. The patient had been wearing soft contact lenses for several years without incident.
She fulfilled the Beighton score(5). Her medical history revealed arthralgia in two joints (right elbow and right wrist) for more than 6 months, two episodes of shoulder dislocation, a marfanoid habitus, myopia, and mitral valve prolapse.
At the time of consultation, her refraction had been stable for more than 3 years, and both topography and pachymetry findings appeared normal. Topography revealed symmetric bow-tie patterns in both eyes. Central ultrasonic pachymetry revealed a corneal thickness of 515 µm and 513 µm in the right and left eyes, respectively. Central keratometry revealed a corneal surface curvature of 42.99/42.24 diopters (D) in the right eye and 42.77/42.18 D in the left eye, representing a topographical cylinder (0.75 and 0.59, respectively; Figures 3 A and 3 B). According to the Randleman customized ectasia risk score, each eye represented a low risk for the development of excimer keratectasia.
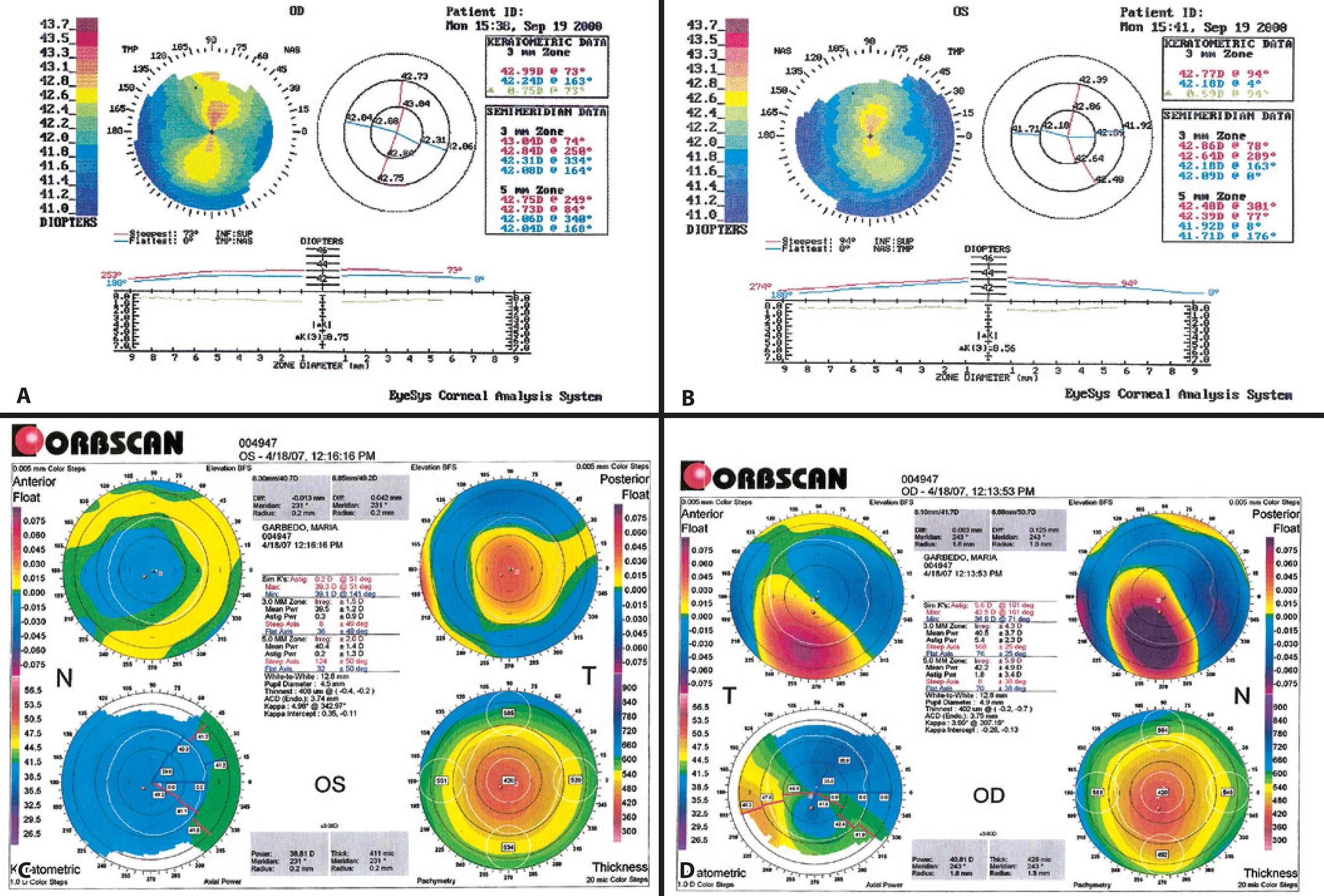
Figure 3 A) Preoperative right topography. B) Preoperative left topography. C) Postoperative right topography. D) Postoperative left topography.
Uneventful LASIK was performed in November 2000 on both eyes, with a 1-week interval between surgeries. The Automated Corneal Shaper microkeratome (Bausch & Lomb) with a 160-µm plate and a Meditec MEL-60 excimer laser was used. In both eyes, the optic ablation zone was 6 mm, and the estimated ablation depth was 43 µm. The intended correction was the manifest refraction in both eyes. Three months after surgery, the uncorrected distance visual acuity (UDVA) was 20/20 in the right eye and 20/20 in the left. Biomicroscopy was clear, and the patient was discharged from the clinic. In May 2006, she returned, reporting that the visual acuity in the right eye had deteriorated over the past 6 months; she reported no problems with the left eye. The UDVA was 20/200 in the right eye and 20/20 in the left eye. The CDVA was 20/25, with a refraction of -4.00 × 70° in the right eye. The intraocular pressure remained normal before and after surgery. With the OCT Visante, the LASIK interface was not visible. The Reichert Ocular Response Analyzer (ORA) showed low values for corneal hysteresis (CH; right eye, 7.4; left eye, 8.5) and corneal resistance factor (CRF; right eye, 6.3; left eye, 6.9). Topography (Orbscan, Bausch & Lomb) of the right eye showed an irregular pattern, suggestive of corneal ectasia (Figure 3 C). Pachymetry revealed a thickness of 402 µm at the thinnest point. The posterior float was 125 µm.
Topography (Orbscan, Bausch & Lomb) of the left eye appeared normal. Pachymetry revealed a thickness of 408 µm at the thinnest point. The posterior float was 42 µm (Figure 3 D).
Because the surgeon believed that it was important to minimize the possible progression of keratectasia while the patient was away, corneal collagen cross-linking with riboflavin and ultraviolet A (IROC, Zurich, Switzerland) irradiation was performed in 2007. Both eyes remained stable during 5 years of follow-up.
DISCUSSION
Corneal ectasia is a serious complication of laser refractive surgery. Progressive distortion of the cornea can lead to a significant decrease in CDVA and may ultimately require transplantation for visual rehabilitation. Although several risk factors have been well outlined, the pathogenesis of the condition is not clearly understood(3). Various authors have attributed age, preoperative corneal pachymetry, residual stromal bed thickness, high preoperative correction, number of enhancements, flap creation, the presence of subclinical corneal disease, or high posterior float on topography to be related to the onset or progression of ectasia(1-3). Our patient's preoperative and intraoperative assessments with no known risk factors suggest that the patient was at a low risk of developing ectasia. Before surgery, CDVA was 20/20 in both eyes. Her refraction was not high and had been stable for several years. Preoperative corneal topography and pachymetry findings appeared normal in both eyes.
Because of the retrospective nature of the case, the preoperative topography maps were not available for further review, and no further comment on the same is possible.
BJHS may be a sign of biomechanical weakness and a possible risk factor for keratectasia.
In conclusion, we described the first case, to the best of our knowledge, of keratectasia after LASIK in both eyes in a patient with BJHS. The right eye developed late-onset ectasia despite the relative absence of presurgical risk factors. We suggest that screening for BJHS as a possible risk factor will limit the incidence of ectasia in these patients and support the decision to proceed with an alternative to LASIK surgery. Alternative surgical options, including surface ablation and phakic intraocular lenses, should be considered patients with BJHS.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

