

Fernanda Pedreira Magalhães1; Luciene Barbosa de Sousa1; Lauro Augusto de Oliveira1
DOI: 10.1590/S0004-27492012000300016
ABSTRACT
Regardless of significant progress in the field of corneal transplantation to treat corneal opacification, some cases of corneal blindness still present a poor prognosis for conventional penetrating keratoplasty. In patients with repeated graft failure and/or with severe ocular surface disease, the Boston type I keratoprosthesis (type I BKPro) has become a viable option. Modifications in its design and postoperative management have improved the long-term outcomes of visual acuity, retention, and postoperative infection rates. These advances made the type I BKPro be considered a safe alternative for visual rehabilitation in many patients with corneal pathologies. However, postoperative handle of chronic comorbidities, such as glaucoma, is still critical for preserving the visual gains achieved with BKPro.
Keywords: Cornea; Corneal diseases; Prostheses and implants; Corneal transplantation; Prosthesis design
RESUMO
Apesar dos avanços consideráveis nas técnicas de transplantes da córnea para o tratamento de opacidades corneanas, alguns casos de cegueira de etiologia corneana ainda apresentam prognóstico reservado com a ceratoplastia penetrante convencional. Nesses pacientes, tais como aqueles vitimas de múltiplas falências após transplante de córnea e/ou com doença da superfície ocular avançada, a ceratoprótese de Boston tipo I (BKPro tipo I) tem se tornado uma opção viável. Modificações no seu modelo e no manejo pós-operatório melhoraram os resultados de acuidade visual, retenção e taxas de infecção pós-operatória a longo prazo. Devido às melhorias no dispositivo, a BKPro tipo I tem sido considerada uma alternativa cirúrgica segura para a reabilitação visual em muitos pacientes com patologias corneanas avançadas. Contudo, o controle pós-operatório de co-morbidades crônicas, como glaucoma, é fundamental para preservar os ganhos visuais obtidos com a BKPro.
Descritores: Córnea; Doenças da córnea; Próteses e implantes; Desenho de prótese
REVIEW ARTICLE ARTIGO DE REVISÃO
Boston type I keratoprosthesis. Review
Ceratoprótese de Boston tipo I. Revisão
Fernanda Pedreira Magalhães; Luciene Barbosa de Sousa; Lauro Augusto de Oliveira
Department of Ophthalmology, Universidade Federal de São Paulo - UNIFESP - São Paulo (SP), Brazil
ABSTRACT
Regardless of significant progress in the field of corneal transplantation to treat corneal opacification, some cases of corneal blindness still present a poor prognosis for conventional penetrating keratoplasty. In patients with repeated graft failure and/or with severe ocular surface disease, the Boston type I keratoprosthesis (type I BKPro) has become a viable option. Modifications in its design and postoperative management have improved the long-term outcomes of visual acuity, retention, and postoperative infection rates. These advances made the type I BKPro be considered a safe alternative for visual rehabilitation in many patients with corneal pathologies. However, postoperative handle of chronic comorbidities, such as glaucoma, is still critical for preserving the visual gains achieved with BKPro.
Keywords: Cornea; Corneal diseases/surgery; Prostheses and implants; Corneal transplantation; Prosthesis design
RESUMO
Apesar dos avanços consideráveis nas técnicas de transplantes da córnea para o tratamento de opacidades corneanas, alguns casos de cegueira de etiologia corneana ainda apresentam prognóstico reservado com a ceratoplastia penetrante convencional. Nesses pacientes, tais como aqueles vitimas de múltiplas falências após transplante de córnea e/ou com doença da superfície ocular avançada, a ceratoprótese de Boston tipo I (BKPro tipo I) tem se tornado uma opção viável. Modificações no seu modelo e no manejo pós-operatório melhoraram os resultados de acuidade visual, retenção e taxas de infecção pós-operatória a longo prazo. Devido às melhorias no dispositivo, a BKPro tipo I tem sido considerada uma alternativa cirúrgica segura para a reabilitação visual em muitos pacientes com patologias corneanas avançadas. Contudo, o controle pós-operatório de co-morbidades crônicas, como glaucoma, é fundamental para preservar os ganhos visuais obtidos com a BKPro.
Descritores: Córnea; Doenças da córnea/cirurgia; Próteses e implantes; Desenho de prótese
INTRODUCTION
The approach of using an artificial material to replace an opaque cornea was first described by Pellier de Quengsy, more than 200 years ago(1).Since that time, several attempts to develop a modern keratoprosthesis to treat patients with corneal blindness and a poor prognosis for penetrating keratoplasty have been developed, with various models using different material, innovative designs and surgical techniques(2,3).
Despite progress in the conception of keratoprosthesis, the heterogeneity of the results related to different devices and the serious complications historically described, hampered the acceptance of the keratoprosthesis as a safe option for surgical treatment. Currently, however, considering modifications made to the designs, and improvements in surgical technique and postoperative management, the keratoprosthesis emerged as a viable alternative in patients at high risk for conventional keratoplasty(4).
One of the most commonly used device is the Boston type I keratoprosthesis (BKPro), developed initially by Dohlman et al., in 1974(5) and approved by the Food and Drug Administration in 1992. Its design and clinical management have undergone several important changes, which increased the BKPro popularity and improved its outcomes in the last several years.
Boston keratoprosthesis devices
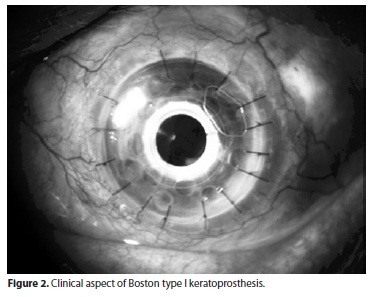
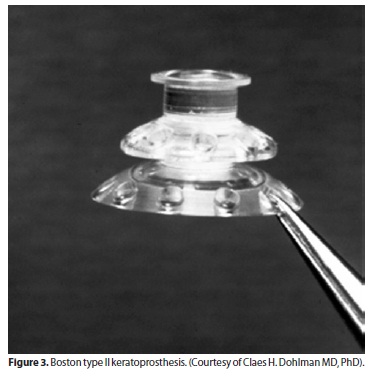
The Boston keratoprosthesis has a collar button design, made of polymethyl methacrylate (PMMA)(2,6).The BKPro has 2 configurations. The type I device, which is the most frequently used type, consists of a PMMA optical front plate and stem, which is inserted through a fresh corneal donor graft, locking into a larger back plate (Figure 1). It is usually recommended for patients with good lid anatomy and blink to preserve a healthy ocular surface(3)(Figure 2). The type II BKPro has a similar design, with an added 2 mm long anterior cylinder that protrudes through the lids or between a tarsorraphy (Figure 3). It is reserved for cicatrizing corneal diseases with poor tear function(2,7).
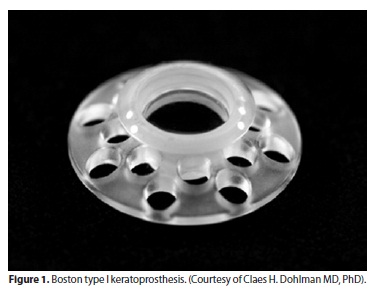
The type I BKPro dioptric power is polished into its front plate power, and the devices differ based on the eye phakic status: one design for aphakia and the other for pseudophakia. The device for aphakia is custom based on the axial length of the recipent eye(8).
Advances in type I BKPro design have improved its retention rate. The latest version approved by the Food and Drug Administration (FDA) for marketing presents 8 holes of 1.3 mm diameter in the back plate to allow aqueous nutrition for the corneal button sandwiched between the front and back plate, protecting the tissue from necrosis and melt(2,9). Moreover, the addition of a titanium locking ring, on the portion of the front plate protruding from the back plate, helps to prevent latter intraocular unscrewing of the BKPro complex. Alternatively, the back plate can also be made of titanium, which seems to cause less postoperative reaction than PMMA(10). The titanium back plate has been studied since 2005 and it has been tried with 8 and 16 holes to allow a better nutrition for the corneal button. However, as far as we know, it has not yet been approved by the FDA for marketing(10).
Indications
The type I BKPro is indicated for patients with refractory corneal blindness and poor prognosis for penetrating keratoplasty. It is usually reserved for patients with multiple graft failure and those with ocular surface disease in which conventional corneal transplantation is considered of high risk(11).
Although the most favorable BKPro indications are multiple failed corneal grafts(12), other ocular conditions have been suitable for BKPro use. Expanding indications include cases of ocular trauma, chemical burns(13), herpetic keratitis(14), aniridia(15), pediatric corneal opacities(16), autoimmune ocular disorders, and severe corneal vascularization(17-20) (Table 1). The expected outcomes are influenced by the primary indication, with best results observed in non-cicatrizing conditions, while patients with autoimmune diseases, such as Stevens-Johnson syndrome (SJS), usually present a less encouraging prognosis(12).
Until recently, taking into account the high risk of postoperative complications, the BKPro implantation was reserved for cases with advanced bilateral disease, and thus, good visual acuity in the fellow eye was considered a relative contra-indication for the procedure(12). However, considering the advances in the postoperative management, reducing drastically the rate of complications(2), the BKPro has now been implanted in patients with unilateral visual impairment with good results in terms of binocular function restoration(4,21).
Surgical procedure
The technique for implanting the type I BKPro has been previously described by Dohlman et al.(22).First of all, a corneal donor button is prepared (8.5 - 9.0 mm) and a central 3 mm hole is trephined. For better BKPro centration the 3 mm central trephination can be performed before the outer diameter punch is used(23). The donor button is then placed over the stem of the front plate, and the back plate is placed on top of this complex. A titanium locking ring is then snapped into place. The recipient cornea is prepared as for traditional penetrating keratoplasty, with a usual host trephine measuring 0.5 mm less in diameter than the donor graft. The donor button is then sutured with multiple interrupted 10-0 nylon stitches(11).
In phakic patients, lens extraction is necessary at the time of BKPro implantation. If pseudophakic, the intraocular lens (IOL) can be left in place or explanted, depending on the IOL stability. If aphakic, a core anterior vitrectomy is generally performed. At the end of the procedure, a soft contact lens is placed.
Postoperative management and complications
Postoperative care includes the use of soft contact lens over the keratoprosthesis in all patients for an indefinite time, with regular replacement. The addition of a continue use of a bandage contact lens over the BKPro has been of great benefit for better hydratation of the exposed tissues, enhancing the device retention and preventing complications as dellen formation, epithelial defects and corneal melt(2).
Postoperatively, all patients need a topical regimen of steroids with dosage tapering over the first weeks. A maintenance dose can be adopted according to levels of intraocular inflammation.
An indefinitely prophylactic antibiotic regimen is recommended for infectious complications prevention. The recommended prophylaxis routine includes the use of daily topical fluoroquinolone drops, with a recent addition of topical 1.4% vancomycin, especially in cases of autoimmune diseases(2).The inclusion of topical vancomycin to the standard prophylactic regimen has dramatically reduced the incidence of bacterial endophthalmitis in BKPro eyes(24).
The continued use of bandage contact lenses and the long-term use of broad-spectrum antibiotics and corticosteroids, however, have the potential of altering the ocular microbiota and predispose fungal colonization. Considering the low rate of fungal infection, there is no literature support for addition of a chronic topical anti-fungal prophylaxis in the routine management of KPro patients, but a short course of topical amphotericin might be considered in patients with proven fungal colonization(25). Also, the addition of monthly topical 5% povidone-iodine in the postoperative care routine might be an alternative for preventing fungal colonization(26).
In the past, the most common complications of a BKPro were melt around the BKPro interface, leakage of aqueous, instability of the device, glaucoma, retinal detachment, infectious keratitis, and the most frightening of all, endophthalmitis(2).
Many of the serious complications are frequently seen in the first year of surgery. The greatest vision-threatening risk facing patients with any type of keratoprosthesis is bacterial endophthalmitis, most cases associated with Gram-positive pathogens(2,24,27).Early recognition and aggressive treatment are crucial for favorable results in theses cases(28). Since 1990, topical antibiotic prophylaxis has been used to prevent bacterial endophthalmitis in patients with the Boston KPro(25). The topical recommend regimen of 0.5% moxifloxacin or 0.3% gatifloxacin associated with 1.4% vancomycin reduced the incidence of endophthalmitis since last decade(2).
Nowadays, one of the most common BKPro problems is the development of a retroprosthetic membrane (RPM), occurring in 25-65% of patients within a year after the device implantation(29). It can usually be treated with yttrium-aluminum-garnet (YAG) laser membranotomy, with few cases requiring a surgical mebranectomy(11).The etiology of RPM is unknown; authors have reported that the performance of other intraocular surgery at the time of keratoprosthesis implantation(4), increased anterior segment inflammation(17), diabetes, hypertension(30), and race(30), increase the risk of RPM formation(31).Results from a Multicenter Study regarding this issue reported that eyes at the highest risk for retroprosthetic membrane development are those receiving corneal replacement for infectious keratitis and aniridia.(y) Stacy et al., characterized retroprosthetic membrane as a fibrous membrane originated from the host's corneal stroma by light microscopy. This data suggest that stromal downgrowth might be the major element of the retro KPro membranes(32).
The visual recovery and long term outcomes of BKPro patients can also be severely limited by retina and glaucoma pathologies. Posterior segment abnormalities may be present prior to BKPro implantation or may occur during or after the surgical procedure(33).The related incidence of retinal detachment after BKPro implantation range from 3.5to8% of cases(11,4). Modified vitreoretinal surgical techniques, due to anatomical difficulties frequently encountered during surgical procedure, can be effectively and safely used to treat posterior segment complications in these patients(33).
Glaucoma can severely compromise visual rehabilitation. The onset and/or progression of glaucoma remain ongoing challenges in the management of patients with BKPro(34).The diagnosis of glaucoma in these patients is made difficult by the absence of a reliable tool to measure the intraocular pressure (IOP), with digital finger palpation being the most used method of estimating the IOP(34).Rigorous perioperative management of elevated IOP is essential for long-term success of BKPro surgery(35).These patients should be followed closely by a multidisciplinary team, including glaucoma and retina specialists. Regular visual fields and photo documentation of the optic nerve should be performed.
Besides medical hypotensive treatment, glaucoma drainage devices or cyclophotocoagulation procedures have been used to address this potential complication(36,37). Considering that standard glaucoma shunts are often susceptible to capsule fibrosis obstructing the tube flow, efforts have been made to produce modified tube devices, such as Ahmed valve shunts to divert aqueous humor to alternative epithelialized cavities (lacrimal sac and ethmoid sinuses) where an obstructing capsule is less likely to form obstructing the flow and consequently increasing the intraocular pressure(38). Randomized studies with longer follow-up, though, are necessary to address concerns about a possible increase incidence of infectious complications and hypotony in theses modified drainage devices.
RESULTS
Several studies have been published to report the outcomes with Boston type I keratoprosthesis (Table 2). Results from the first multicenter study, including 136 eyes with this device, were relevant diffusing this surgical technique, demonstrating improvements in the retention rate (95% in 8.5 months of follow-up) and in the visual prognosis(11). The main postoperative complication observed was RPM formation (25%), followed by an increase of IOP (15%). There were no reported cases of endophthalmitis after surgery. Considering the study group preoperative characteristics, better outcomes were observed in patients previously diagnosed with multiple graft failure and chemical burns, compared with patients with cicatricial conjunctivitis (SJS and OCP).
Aldave et al., (4) found similar results with type I BKPro regarding anatomical retention and visual acuity improvement in 50 eyes of 49 patients. The most common complications were RPM (44%) and persistent epithelial defects (38%). In this study, with an average follow-up of 17 months, there were no cases of endophthalmitis. In 2010, Dunlap et al., reported good short-term visual outcomes of BKPro implantation in 126 eyes(39). Eighty-two percent of these cases improved visual acuity within 6 months after surgery, with mean spherical refractive error of 0.57 diopters (D) and a mean astigmatism of 0.10 D.
Bradley et al.(40), analyzed 30 eyes of 28 patients undergoing implantation of type I BKPro with a mean follow-up of 19 months. The anatomical retention rate was 81.3% and final visual acuity ≥ 20/200 observed in 77% of eyes. Glaucoma and posterior segment pathologies were the main causes of poor visual acuity improvement. RPM was also considered the most frequent complication (43%). Three patients (10%) had endophthalmitis despite the correct use of postoperative medications (including 1.4% vancomycin eye drops). At the same center, in a longer follow-up, Greiner et al.(41), found that a significant number of patients can decrease vision during the postoperative course. End-stage glaucoma was the most commonly cause of visual loss when best corrected visual acuity (BCVA) > 20/200 was not retained.
Future perspectives
Considering the important reduction in acute complications related to type I BKPro implantation, attention should be focused on its long-term visual outcomes. Currently, BKPro results are still limited by chronic comorbidities, such as glaucoma. Improvements and developments on IOP measurements devices are necessary. Several attempts for new methods to monitor IOP have been made. Telemetric IOP measurement allows monitoring of IOP in patients who cannot be measured by current methods, such as patients with keratoprosthesis. A recent experimental study of telemetric IOP records used a wireless transducer implanted in rabbits after extracapsular lens extraction demonstrating concordance with direct manometry measures(42). Further studies are necessary, however, to validate its use and safety in human eyes.
Besides the significant impact of a broad-spectrum antibiotic routine in reducing infectious complications related to BKPro patients, the need of using an indefinite postoperative eye drops regimen may be limited by patients' adherence and compliance. The creation of drug delivery systems such as a contact lens slow release of antibiotic might be a future option to solve this problem(43).
Another point to be addressed is biocompatibility. Although the favorable retention rates demonstrated in several case series are encouraging, other materials such as titanium seems to be less problematic to corneal cells, allowing a better interaction and less inflammation(44). Behlau et al,, reported the potential of improving the biocompatibility and the potential of inhibition of bacterial biofilm bonding N,N-hexyl,methyl-polyethylenimine to BKPro(45). Wang et al., also reported encouraging biointegration results coating polymethyl methacrylate with hidroxyapatite. They demonstrated an enhanced keratocyte proliferation in porcine cornas ex vivo(46).
In summary, over the last few decades, important advances in the field of keratosprosthesis implantation were demonstrated. Despite of that, for good long-term results, it is imperative that a multidisciplinary team carefully monitor all patients submitted to this procedure, with regular visits and prompt support considering any kind of complications. Future studies, attesting the very long-term stability and visual maintenance are still pending. At this point, however, the literature results are very encouraging to use the type I BKPro as a viable option for visual rehabilitation in patients with advanced corneal disease.
REFERENCES
1. Pellier de Quengsy G. Precis au cours d'operations sur la chirurgie des yeux. Paris: Didot; 1789.
2. Khan BF, Harissi-Dagher M, Khan DM, Dohlman CH. Advances in Boston keratoprosthesis: enhancing retention and prevention of infection and inflammation. Int Ophthalmol Clin. 2007;47(2):61-71.
3. Gomaa A, Comyn O, Liu C. Keratoprosthesis in clinical practice - a review. Clin Experiment Ophthalmol. 2010;38(2):211-24.
4. Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116(4):640-51.
5. Dohlman CH, Schneider HA, Doane MG. Prosthokeratoplasty. Am J Ophthalmol. 1974; 77(5):694-700.
6. Ma JJ, Graney JM, Dohlman CH. Repeat penetrating keratoplasty versus the Boston keratoprosthesis in graft failure. Int Ophthalmol Clin. 2005;45(4):49-59.
7. Pujari S, Siddique SS, Dohlman CH, Chodosh J. The Boston keratoprosthesis type II: The Massachusetts Eye and Ear Infirmary experience. Cornea. 2011;30(12):1298-303.
8. Utine CA, Tzu J, Dunlap K, Akpek EK. Visual and clinical outcomes of explantation versus preservation of the intraocular lens during keratoprosthesis implantation. J Cataract Refract Surg. 2011;37(9):1615-22.
9. Harissi-Dagher M, Khan BF, Schaumberg DA, Dohlman CH. Importance of nutrition to corneal grafts when used as a carrier of the Boston Keratoprosthesis. Cornea. 2007;26(5):564-8.
10. Todani A, Ciolino JB, Ament JD, Colby KA, Pineda R, Belin MW, et al. Titanium back plate for a PMMA keratoprosthesis: clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1515-8.
11. Zerbe BL, Belin MW, Ciolino JB. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113(10):1779-84.
12. Yaghouti F, Nouri M, Abad JC, Power WJ, Doane MG, Dohlman CH. Keratoprosthesis: preoperative prognostic categories. Cornea. 2001;20(1):19-23.
13. Harissi-Dagher M, Dohlman CH. The Boston Keratoprosthesis in severe ocular trauma. Can J Ophthalmol. 2008;43(2):165-9.
14. Khan BF, Harissi-Dagher M, Pavan-Langston D, Aquavella JV, Dohlman CH. The Boston Keratoprosthesis in herpetic keratitis. Arch Ophthalmol. 2007;125(6):745-9.
15. Akpek EK, Harissi-Dagher M, Petrarca R, Butrus SI, Pineda R 2nd, Aquavella JV, et al. Outcomes of Boston Keratoprosthesis in aniridia: a retrospective multicenter study. Am J Ophthalmol. 2007;144(2):227-31.
16. Aquavella JV, Gearinger MD, Akpek EK, McCormick GJ. Pediatric keratoprosthesis. Ophthalmology. 2007;114(5):989-94.
17. Sayegh RR, Ang LP, Foster CS, Dohlman CH. The Boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol. 2008;145(3):438-44.
18. Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30(11):1187-94.
19. Ciralsky J, Papaliodis GN, Foster CS, Dohlman CH, Chodosh J. Keratoprosthesis in autoimmune disease. Ocul Immunol Inflamm. 2010;18(4):275-80.
20. Colby KA, Koo EB. Expanding indications for the Boston keratoprosthesis. Curr Opin Ophthalmol. 2011;22(4):267-73.
21. Pineles SL, Ela-Dalman N, Rosenbaum AL, Aldave AJ, Velez FG. Binocular visual function in patients with Boston type I keratoprostheses. Cornea. 2010;29(12):1397-400.
22. Dohlman CH, Abad JC, Dudenhoefer EJ, Graney JM. Keratoprosthesis: beyond corneal graft failure. In: Spaeth GL, editor. Ophthalmic surgery: principles and practice. 3 ed. Philadelphia: W.B. Saunders; 2002. p.199-207.
23. Khalifa YM, Moshirfar M. Improved centration of the type 1 Boston Keratoprosthesis in donor carrier tissue. Clin Ophthalmol. 2010;4:931-3.
24. Durand ML, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea. 2009;28(8):896-901.
25. Barnes SD, Dohlamn CH, Durand ML. Fungal colonization and infection in Boston keratoprosthesis. Cornea. 2007;26(1):9-15.
26. Magalhães FP, Nascimento HM, Ecker DJ, Lowery K, Sampath R, Rosenblatt MI, et al. Microbiota evaluation of patients with a Boston type I keratoprosthesis treated with topical 0.5% moxifloxacin and 5% povidone-iodine. Cornea. 2012 (Epub ahead of print).
27. Nouri M, Terada H, Alfonso EC, Foster CS, Durand ML, Dohlman CH. Endophthalmitis after keratoprosthesis. Incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119(4):484-9.
28. Fintelmann RE, Maguire JI, Ho AC, Chew HF Ayres BD. Characteristics of endophthalmitis in patients with the Boston keratoprosthesis. Cornea. 2009;28(8):877-8. Comment in: Characteristics of endophalmitis with Boston keratoprosthesis. Cornea. 2012.
29. Stacy RC, Jakobiec FA, Michaud NA, Dohlman CH, Colby KA. Characterization of retrokeratoprosthetic membranes in the Boston type 1 keratoprosthesis. Arch Ophthalmol. 2011;129(3):310-6.
30. Hicks CR, Hamilton S. Retroprosthetic membranes in Alpha-Cor patients: risk factors and prevention. Cornea. 2005;24(6):692-8.
31. Rudnisky CJ, Belin MW, Todani A, Al-Arfaj K, Ament JD, Zerbe BJ, Ciolino JB; Boston Type 1 Keratoprosthesis Study Group. Risk factors for the development of retroprosthetic membranes with Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. 2012;119(5):951-5.
32. Stacy RC, Jakobiec FA, Michaud NA, Dohlman CH, Colby KA. Characterization of retrokeratoprosthetic membranes in the Boston type 1 keratoprosthesis. Arch Ophthalmol. 2011;129(3):310-6.
33. Ray S, Khan BF, Dohlman CH, D'Amico DJ. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol. 2002;120(5):559-66.
34. Banitt M. Evaluation and management of glaucoma after keratoprosthesis. Curr Opin Ophthalmol. 2011;22(2):133-6.
35. Kamyar R, Weizer JS, de Paula FH, Stein JD, Moroi SE, John D, et al. Glaucoma associated with Boston type I keratoprosthesis. Cornea. 2012;31(2):134-9.
36. Li JY, Greiner MA, Brandt JD, Lim MC, Mannis MJ. Long-term complications associated with glaucoma drainage devices and Boston keratoprosthesis. Am J Ophthalmol. 2011;152(2):209-18.
37. Talajic JC, Agoumi Y, Gané S, Moussally K, Harissi-Dagher M. Prevalence, progression, and impact of glaucoma on vision after Boston type 1 keratoprosthesis surgery. Am J Ophthalmol. 2012;153(2):267-74.
38. Dohlman CH, Grosskreutz CL, Chen TC, Pasquale LR, Rubin PA, Kim EC, et al. Shunts to divert aqueous humor to distant epithelialized cavities after keratoprosthesis surgery. J Glaucoma. 2010;19(2):111-5.
39. Dunlap K, Chak G, Aquavella JV, Myrowitz E, Utine CA, Akpek E. Short-term visual outcomes of Boston type 1 keratoprosthesis implantation. Ophthalmology 2010; 117(4):687-92.
40. Bradley JC, Hernandez EG, Schawb IR, Mannis MJ. Boston type I keratoprosthesis: the University of California Davis experience. Cornea. 2009;28(3):321-7.
41. Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston Type 1 keratoprosthesis at the University of California, Davis experience. Ophthalmology. 2011;118(8):1543-50.
42. Todani A, Behlau I, Fava MA, Cade F, Cherfan DG, Zakka FR, et al. Intraocular pressure measurement by radio wave telemetry. Invest Ophthalmol Vis Sci. 2011;52(13): 9573-80.
43. Ciolino JB, Dohlman CH, Kohane DS. Contact lenses for drug delivery. Semin Ophthalmol. 2009;24(3):156-60.
44. Ament JD, Spurr-Michaud SJ, Dohlman CH, Gipson IK. The Boston Keratoprosthesis: comparing corneal epithelial cell compatibility with titanium and PMMA. Cornea. 2009;28(7):808-11.
45. Behlau I, Mukherjee K, Todani A, Tisdale AS, Cade F, Wang L, et al. Biocompatibility and biofilm inhibition of N,N-hexyl,methyl-polyethylenimine bonded to Boston Keratoprosthesis materials. Biomaterials. 2011;32(34):8783-96.
46. Wang L, Jeong KJ, Chiang HH, Zurakowski D, Behlau I, Chodosh J, et al. Hydroxyapatite for keratoprosthesis biointegration. Invest Ophthalmol Vis Sci. 2011;52(10):7392-9.
 Correspondence address:
Correspondence address:
Fernanda Pedreira Magalhães.
Department of Ophthalmology, Universidade Federal de São Paulo.
Rua Botucatu, 820 - São Paulo (SP) - 04023-062 - Brazil
E-mail: [email protected]
Submitted for publication: April 26, 2012
Accepted for publication: June 14, 2012
Study was carried out at Department of Ophthalmology, Universidade Federal de São Paulo.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: F.P.Magalhães, None; L.B.Sousa, None; L.A.Oliveira, None.