

Carolina Pelegrini Barbosa1; Francisco Rosa Stefanini1; Fernando Penha1; Miguel Ângelo Góes2; Sérgio Antonio Draibe2; Maria Eugênia Canziani2; Augusto Paranhos Junior1
DOI: 10.1590/S0004-27492011000200007
ABSTRACT
AIM: To evaluate the intraocular pressure and ocular perfusion pressure during a hemodialysis. METHODS: Sixty-seven eyes from thirty-five patients were evaluated at the beggining of hemodialysis, 2 hours and 4 hours after initiation. Intraocular pressure was evaluated using a Tonopen. Systolic and diastolic arterial pressures were measured with a manual sphygmomanometer. The ocular perfusion pressure was estimated by mea suring the difference between 2/3 of the mean arterial pressure and the intraocular pressure values. Generalized estimating equations were used to evaluate the difference between the repeated measurements using the appropriate correction for inter-eye dependency. RESULTS: There was no statistically significant difference in ocular perfusion pressure, in intraocular pressure (p=0.93) and in systolic arterial pressure (p=0.92) at the three time points (p=0.69). But, when analyzing the extreme values, some patients exhibited lower diastolic perfusion pressures at all time points. CONCLUSION: Our results did not support the view that significant changes in ocular perfusion pressure and intraocular pressure occur during hemodialysis session. However, we observed that some patients exhibited lower diastolic perfusion pressures, which could be a poor prognostic factor for glaucoma patients.
Keywords: Renal dialysis; Renal insufficiency, chronic; Intraocular pressure; Glaucoma
RESUMO
OBJETIVO: Avaliar a pressão intraocular e a pressão de perfusão ocular durante uma sessão de hemodiálise. MÉTODOS: Sessenta e sete olhos de trinta e cinco pacientes foram avaliados no início, após 2 horas e após 4 horas do início de uma sessão de hemodiálise. A pressão intraocular foi avaliada usando o aparelho Tonopen. Pressões arteriais sistólica e diastólica foram aferidas usando esfigmomanômetro manual. A pressão de perfusão ocular foi estimada por meio do cálculo da diferença entre 2/3 da pressão arterial média e valores da pressão intraocular. Equações de estimação generalizada foram usadas para avaliar a diferença entre medidas repetidas usando correção apropriada para dependência entres olhos. RESULTADOS: Não houve diferença estatisticamente significativa na pressão de perfusão ocular, PIO (p=0,93) e na pressão arterial sistólica (p=0,92) nos três períodos medidos da hemodiálise (p=0,69). Mas, quando analisados valores extremos, alguns pacientes exibiram pressões diastólicas menores em todos os períodos aferidos. CONCLUSÃO: Nossos resultados não apontaram mudanças significativas na pressão de perfusão ocular e pressão intraocular durante a hemodiálise. No entanto, foi observado que alguns pacientes exibiram pressões diastólicas menores, o que pode ser um fator de prognóstico ruim para pacientes com glaucoma.
Descritores: Diálise renal; Insuficiência renal crônica; Pressão intraocular; Glaucoma
ORIGINAL ARTICLES
Intraocular pressure and ocular perfusion during hemodialysis
Pressão intraocular e perfusão ocular durante hemodiálise
Carolina Pelegrini BarbosaI; Francisco Rosa StefaniniI; Fernando PenhaI; Miguel Ângelo GóesII; Sérgio Antonio DraibeII; Maria Eugênia CanzianiII; Augusto Paranhos JuniorI
IPhysician, Ophthalmology Department, Universidade Federal de São Paulo - UNIFESP - São Paulo (SP), Brazil
IIPhysician, Division of Nephrology, Universidade Federal de São Paulo - UNIFESP - São Paulo (SP), Brazil
ABSTRACT
AIM: To evaluate the intraocular pressure and ocular perfusion pressure during a hemodialysis.
METHODS: Sixty-seven eyes from thirty-five patients were evaluated at the beggining of hemodialysis, 2 hours and 4 hours after initiation. Intraocular pressure was evaluated using a Tonopen. Systolic and diastolic arterial pressures were measured with a manual sphygmomanometer. The ocular perfusion pressure was estimated by mea suring the difference between 2/3 of the mean arterial pressure and the intraocular pressure values. Generalized estimating equations were used to evaluate the difference between the repeated measurements using the appropriate correction for inter-eye dependency.
RESULTS: There was no statistically significant difference in ocular perfusion pressure, in intraocular pressure (p=0.93) and in systolic arterial pressure (p=0.92) at the three time points (p=0.69). But, when analyzing the extreme values, some patients exhibited lower diastolic perfusion pressures at all time points.
CONCLUSION: Our results did not support the view that significant changes in ocular perfusion pressure and intraocular pressure occur during hemodialysis session. However, we observed that some patients exhibited lower diastolic perfusion pressures, which could be a poor prognostic factor for glaucoma patients.
Keywords: Renal dialysis; Renal insufficiency, chronic; Intraocular pressure; Glaucoma
RESUMO
OBJETIVO: Avaliar a pressão intraocular e a pressão de perfusão ocular durante uma sessão de hemodiálise.
MÉTODOS: Sessenta e sete olhos de trinta e cinco pacientes foram avaliados no início, após 2 horas e após 4 horas do início de uma sessão de hemodiálise. A pressão intraocular foi avaliada usando o aparelho Tonopen. Pressões arteriais sistólica e diastólica foram aferidas usando esfigmomanômetro manual. A pressão de perfusão ocular foi estimada por meio do cálculo da diferença entre 2/3 da pressão arterial média e valores da pressão intraocular. Equações de estimação generalizada foram usadas para avaliar a diferença entre medidas repetidas usando correção apropriada para dependência entres olhos.
RESULTADOS: Não houve diferença estatisticamente significativa na pressão de perfusão ocular, PIO (p=0,93) e na pressão arterial sistólica (p=0,92) nos três períodos medidos da hemodiálise (p=0,69). Mas, quando analisados valores extremos, alguns pacientes exibiram pressões diastólicas menores em todos os períodos aferidos.
CONCLUSÃO: Nossos resultados não apontaram mudanças significativas na pressão de perfusão ocular e pressão intraocular durante a hemodiálise. No entanto, foi observado que alguns pacientes exibiram pressões diastólicas menores, o que pode ser um fator de prognóstico ruim para pacientes com glaucoma.
Descritores: Diálise renal; Insuficiência renal crônica; Pressão intraocular; Glaucoma
INTRODUCTION
Hemodialysis (HD) is an effective treatment for end-stage renal disease (ESRD). Although HD is a relatively safe procedure, several complications could still arise, and acute complications commonly occur during routine HD treatments(1-2). These include hypotension (25-55% of cases), cramps (5-20%), nausea and vomiting (5-15%), headache (5%), back pain (2-5%), chest pain (2-5%), itchiness (<5%), and fever and chills (<1%)(3). More serious complications include cardiac arrhythmia, convulsion, gaseous embolism, and intracranial hemorrhage(3).
Arterial hypotension still represents a major complication resulting from HD therapy; it frequently shortens the duration of dialysis and increases patient mortality rates(4-5). The pathogenesis of hemodynamic instability in response to fluid removal probably involves many factors such as defective plasma refilling(6), abnormal body heating(7-8), an imbalance between endothelin and nitric oxide synthesis(9), and defective adjustment of arteriovenous tone in response to the changes in the effective plasma volume(10). An abrupt hypovolemia should induce a sympathetic activation that increases the heart rate and total peripheral resistance(10). This in turn prevents an imbalance between the vascular capacitance and plasma underfilling, ultimately resulting in the maintenance of arterial pressure(10).
Many complications from the development of hypotension during and after HD have been reported for adults(11), such as anterior ischemic optic neuropathy (AION) and non-occlusive mesenteric ischemia, which is the major cause of acute abdominal injury in HD patients(12). Intensive ultrafiltration is usually the factor that precipitates hypotension in this patient population(12).
The hypothesis that hypoperfusion of the optic nerve head is a risk factor for glaucoma is controversial. Several epidemiological studies have revealed a strong correlation between glaucoma damage and the presence of low diastolic arterial pressure, which gives rise to inadequate ocular perfusion pressure (OPP)(13-14).
Alterations of OPP could cause ischemia: a poor irrigation of tissues in the optic nerve, thus having deleterious effects. These could be significant reasons for the onset of glaucoma, an optic neuropathy of unknown origin, which presents a distinctive pattern of nerve changes and visual field loss. One explanation for this pathogenesis concerns the "vascular hypothesis" based on the fact that abnormal perfusion of the optic disc would be a major cause of glaucomatous damage(15).
In this study, we evaluate the intraocular pressure, ocular perfusion pressure, systolic and diastolic arterial pressures in patients with end-stage renal disease during hemodialysis.
METHODS
STUDY DESIGN
We performed an observational case series study on 67 eyes from 35 patients with end-stage renal disease (ESRD) during a maintenance HD session in "Rim e Hipertensão" Hospital at the Federal University of São Paulo, Brazil. HD patients received dialysis therapy for at least three months and were dialyzed three times a week for 3-5 hours per session. All patients had arteriovenous fistulae and used a polysulfone hollow fiber dialyzer (F8- Fresenius®).
Exclusion criteria included: iridotomy; diagnosis of glaucoma or other ophthalmological disorder that might affect the evaluated parameters and patients with positive serological tests for human immunodeficiency virus, hepatitis B virus, or hepatitis C virus. Complete ophthalmologic exam was performed. All patients underwent visual acuity measurements with Snellen chart, biomicroscopic examination using slit lamp, fundoscopy and intraocular pressure measurement (IOP).
IOP was measured using a Tonopen (Medtronic Solan TONO PEN XL Applanation tonometer, Joacksonville - Florida, USA), which is a portable electronic applanation device. The IOP was measured at three different times during the HD session: at the beginning, 2 hours after initiation, and 4 hours later at the end of the session. Systolic arterial pressure, diastolic arterial pressure, and body weight were also measured before and after HD. OPP was estimated by measuring the difference between 2/3 of the mean arterial pressure and the IOP values. Mean arterial pressure measurements, which provide indication of overall circulatory pressure load, were estimated by the formula: diastolic arterial pressure + 1/3 (systolic pressure - diastolic pressure).
We considered the guidelines of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High arterial Pressure for this research, which describe normal arterial pressure as systolic arterial pressure < 120 mmHg and diastolic arterial pressure < 80 mmHg(16). We considered for this research low diastolic arterial pressure as < 60 mmHg as another study(17).
All participants signed an informed consent form, which was approved by the local human research ethics committee.
STATISTICAL ANALYSIS
Continuous variables were expressed as mean ± standard deviation or as a percentage. Comparisons between the systolic arterial pressure (SAP) at the three time points during the HD session were evaluated using repeated measures analysis of variance (ANOVA). IOP and OPP at the three time points were analyzed using generalized estimating equations (GEE) models to account for the dependence between eyes from the same patient. SPSS 17.0 for Windows (SPSS Inc, Chicago, Illinois, USA) was used for statistical analysis. All probabilities (p-values) were considered to be statistically significant if they were less than 0.05.
RESULTS
This study included 67 eyes from 35 patients (65.7% male and 34.3% female). The study group was of an average age of 49 ± 17 years (Table 1). The predominant etiologies of ESRD were glomerulonephritis, diabetes mellitus and hypertension (Table 2). The average duration of HD treatment was 63 ± 62 months (range; 1 to 288 months).

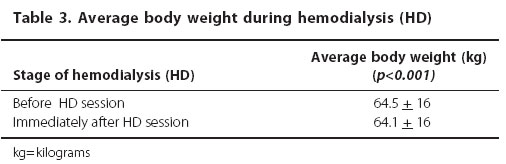
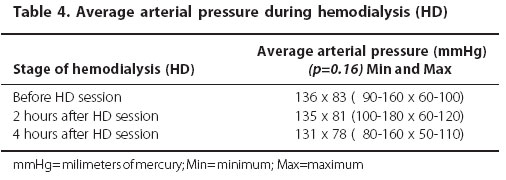
The body weight significantly decreased during the HD session (p<0.001) (Table 3). There was no significant variation in the average arterial pressure during the HD session (p=0.16) (Table 4).
During HD, the IOP and OPP did not significantly vary (p=0.93 and 0.69, respectively) (Figure 1 and 2). There was no correlation between the IOP and SAP variations (p=0.92).
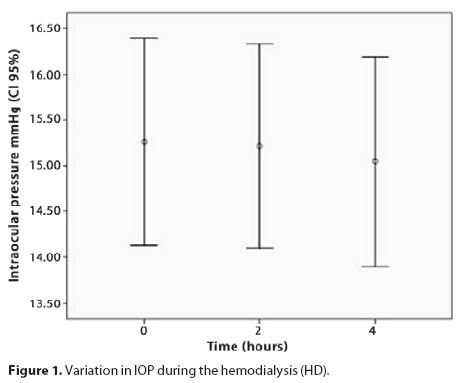
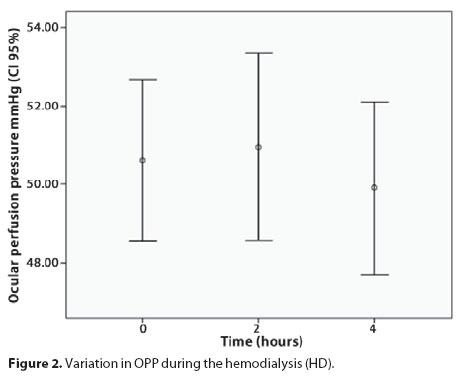
We observed that three patients exhibited lower diastolic perfusion pressure, which is a poor prognostic factor for glaucoma patients but we did not find significant changes in OPP in ESRD patients during a HD session, because average arterial pressure and IOP did not change during this period. No patient presented low diastolic pressure at the beginning of dialysis session.
DISCUSSION
Established risk factors for primary open-angle glaucoma include evidence of elevated IOP, advanced age, race, and a positive family history. Systemic hypertension, low arterial pressure, and the presence of other vascular diseases have also been proposed as risk factors; however, their contribution is controversial and current data are inconclusive(18-19). Results from clinical trials, including the Early Manifest Glaucoma Trial, suggest a potential role for vascular factors in glaucoma progression, given the positive associations with low ocular systolic perfusion pressure (as calculated from the arterial pressure value minus the IOP value), low systolic arterial pressure, and cardiovascular disease history(18).
Several groups have reported that low systemic arterial pressure is a risk factor for glaucoma(20-24). Some authors reported that more advanced disease, lower systolic arterial pressure, and increased IOP are the three main risk factors for disease progression(23). Other authors reported that lower diastolic arterial pressure is a significant risk factor for glaucoma(24). Both the Barbados Eye Study and the Franmingham Eye Study(14,25) suggested that people with field defects have significantly decreased arterial pressure/IOP ratios. These epidemiological studies have revealed a strong correlation between anatomicofunctional glaucoma damage and the presence of low diastolic arterial pressure, which collectively contribute to inadequate OPP(14, 17).
Nowadays, the concept of OPP and its identification of this as an important risk factor for the development and progression of glaucoma have brought together the vascular and mechanical components of glaucoma. Costa et al., believe that it is the balance between IOP and arterial pressure, influenced by the autoregulatory capacity of the eye that determine whether an individual will develop optic disc damage or not(26).
Some authors suggested that glaucoma patients with an exaggerated nocturnal reduction in systemic arterial pressure have an altered OPP outside the period of arterial pressure reduction, which is possibly related to a general vascular dysregulation(27).
In addition to a circadian rhythm for arterial pressure where we observe a nocturnal fall, we also have some situations during the HD session in which we see a reduction in the average arterial pressure value(4-5). Therefore, there is hypotension during the HD session. Many studies were developed to evaluate whether HD induces considerable changes in the IOP(28). Doshiro et al., evaluated the change in IOP during HD and determined that the IOP significantly decreased during HD(29). Some studies have reported that the hypotension following dialysis could cause ischemic optic neuropathy (AION) both in children and adults. AION is an ischemic injury of the optic nerve head caused by hypoperfusion of the posterior ciliary arteries(30).
The present study demonstrates that there is no significant change in IOP or arterial pressure variation during the HD session. Therefore, the OPP did not change either. However, some patients showed a change in intraocular hypertension during the second hour of HD. Some studies have speculated that the IOP in glaucoma patients during HD would increase because of impaired outflow facility(29); or because glaucoma patients show abnormal autoregulation(31). The reasons for abnormal autoregulation in this disease are largely unknown, but there is evidence that reducing IOP improves autoregulation in glaucoma patients(31). However, we did not observe this result in our study because we did not have any patients with glaucomatous disease.
Perfusion pressure and vascular resistance determine arterial pressure. Hemodialysis vascular resistance can change because of the rheological properties of the arterial, so this can change arterial pressure. But in this research the results have considered these properties as many other researches(29,32). Ultimately, in this research, 8% of the patients had hypertension and they were using medications for this disease. It is true that some drugs could interpose with the results but as many other researches we do not know if these drugs can interfere in the results and we assumed that not only hypertensive patients were using drugs but also those with diabetes and other pathologies.
Another limitation in this research is that all the methodology was made measuring the OPP based on systemic arterial pressure and intraocular pressure (considering braquial artery as intraocular pressure) but studies in human models offer some disadvantages, because it is hard to manipulate perfusion pressure(31). Firstly, because several methods available to measure ocular arterial flow cannot be applied in humans due to their invasive nature; secondly, because humans have autoregulation mechanism(31). This is the ability of a vascular bed to adapt its vascular resistance to with this background, calculated OPP cannot be the same as real OPP. When an individual is sitting or standing, the ocular arterial pressure is lower than the brachial arterial pressure because of the effect of gravity (the hydrostatic column effect)(33). But, in this research, all patients assumed the same position, they did not change during the exam in an effort to minimize those effects.
CONCLUSION
Our results did not support the view that significant changes in ocular perfusion pressure or intraocular pressure occur during hemodialysis. However, we observed that some patients exhibited lower diastolic perfusion pressures, which could be a poor prognostic factor for glaucoma patients.
ACKNOWLEDGEMENT
We are grateful to Lady Aurea Garibaldi who helped us with the grammatical review.
REFERENCES
1. Milinkovic M, Zidverc-Trajkovic J, Sternic N, Trbojevic-Stankovic J, Maric I, Milic M, et al. Hemodialysis headache. Clin Nephrol. 2009;71(2):158-63.
2. Voroneanu L, Covic A. Arrhythmias in hemodialysis patients. J Nephrol. 2009;22(6):716-25.
3. Bregman H, Daugirdas J, Ing T. Complications during hemodialysis. In: Daugirdas J, INg TS, editors. Handbook of Dialysis. New York: Little, Brown;1994. p.149.
4. Tisler A, Akocsi K, Borbás B, Fazakas, Ferenczi S, Gorogh S, et al. The effect of frequent or occasional dialysis associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant. 2004;18(12):2601-5.
5. Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3): 1212-20.
6. Mitra S, Chamney P, Greenwood R, Farrington K. Linear decay of relative blood volume during ultrafiltration predicts hemodynamic instability. Am J Kidney Dis. 2002;40(3):556-65.
7. Leunissen KM, Kooman JP, van der Sande FM, van Kuijk WH. Hypotension and ultrafiltration physiology in dialysis. Blood Purif. 2000;18(4):251-4.
8. Maggiore Q, Pizzarelli F, Sisca S, Zoccali C, Parlongo S, Nicolo F, et al. Blood temperature and vascular stability during hemodialysis and hemofiltration. Trans Am Soc Artif Intern Organs. 1982;28:523-7.
9. Raj DS, Vincent B, Simpson K, Sato E, Jones KL, Welbourne TC, et al. Hemodynamic changes during hemodialysis: role of nitric oxide and endothelin. Kidney Int. 2002;61(2):697-704.
10. Kooman JP, Gladziwa U, Bocker G, van Bortel LM, van Hooff JP, Leunissen KM. Role of the venous system in hemodynamics during ultrafiltration and bicarbonate dialysis. Kidney Int. 1992;42(3):718-26.
11. Servilla KS, Groggel GC. Anterior ischemic optic neuropathy as a complication of hemodialysis. Am J Kidney Dis. 1986;8(1):61-3.
12. Brener ZZ, Bergman M, Ohm HK, Winchester JF. Acute non-occlusive mesenteric ischemia of the small bowel in a patient started on hemodialysis: a case report. Cases J. 2008;1(1):217.
13. Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. 1995; 113(2):216-21.
14. Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113(7):918-24. Comment in: Arch Ophthalmol. 1996;114(2):235.
15. Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol. 2009;20(2):73-8.
16. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-72. Erratum in: JAMA. 2003;290(2):197
17. Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000; 107(7):1287-93.
18. Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965-72.
19. Skuta GL, Cantor LB, Weiss JS. Basic and clinical science course. Glaucoma. San Francisco: American Academy of Ophthalmology. 2008.
20. Drance SM, Sweeney VP, Morgan RW, Feldman F. Studies of factors involved in the production of low tension glaucoma. Arch Ophthalmol. 1973;89(6):457-65.
21. Drance SM. Some factors in the production of low tension glaucoma. Br J Ophthalmol. 1972;56(3):229-42.
22. Goldberg I, Hollows FC, Kass MA, Becker B. Systemic factors in patients with low-tension glaucoma. Br J Ophthalmol. 1981;65(1):56-62.
23. Gramer E, Leydhecker W. [Glaucoma without ocular hypertension. A clinical study]. Klin Monbl Augenheilkd. 1985;186(4):262-7. German.
24. Richler M, Werner EB, Thomas D. Risk factors for progression of visual field defects in medically treated patients with glaucoma. Can J Ophthalmol. 1982;17(6):245-8.
25. Leske MC, Podgor MJ. Intraocular pressure, cardiovascular risk variables, and visual field defects. Am J Epidemiol. 1983;118(2):280-7.
26. Costa VP, Arcieri ES, Harris A. Blood pressure and glaucoma. Br J Ophthalmol. 2009;93(10): 1276-82.
27. Gherghel D, Orgul S, Gugleta K, Flammer J. Retrobulbar blood flow in glaucoma patients with nocturnal over-dipping in systemic blood pressure. Am J Ophthalmol. 2001;132(5):641-7.
28. Lifshitz T, Levy J, Cagnano E, Halevy S. Severe conjunctival and eyelid involvement in pemphigus vulgaris. Int Ophthalmol. 2004;25(2):73-4.
29. Doshiro A, Ban Y, Kobayashi L, Yoshida Y, Uchiyama H. Intraocular pressure change during hemodialysis. Am J Ophthalmol. 2006;142(2):337-9.
30. Kim JS, Deputy S, Vives MT, Aviles DH. Sudden blindness in a child with end-stage renal disease. Pediatr Nephrol. 2004;19(6):691-3.
31. Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow - Relevance for glaucoma. Exp Eye Res. 2010 Sep 22. [Epub ahead of print] .
32. Schulman G. Complications of hemodialysis. In: Jacobson HR, Striker GE, Klahr S. The priniciples and practice of nephrology. Philadelphia: B-C Decker; 1991. cap 127; p.757-65.
33. Caprioli J, Coleman AL; Blood Flow in Glaucoma Discussion. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol. 2010;149(5):704-12.
 Correspondence address:
Correspondence address:
Carolina Pelegrini Barbosa.
Rua Botucatu, 821
São Paulo - SP
CEP: 04023-062
Brazil
E-mail: [email protected]
Submitted for publication: December 2, 2010
Accepted for publication: March 30, 2011
Studycarried out at the Departamento de Oftalmologia, Universidade Federal de São Paulo - UNIFESP.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: C.P.Barbosa, None; F.R.Stefanini, None; F.Penha, None; M.A.Góes, None; S.A.Draibe, None; M.E.Canziani, None; A.Paranhos Junior, None.