

Rafael Vidal Mérula1; Sebastião Cronemberger1; Alberto Diniz Filho1; Nassim Calixto1
DOI: 10.1590/S0004-27492008000600005
ABSTRACT
PURPOSE: To compare morphometric features between fellow acute primary angle-closure (APAC) eyes and glaucomatous or suspect eyes with narrow angle (NA). METHODS: Fellow eyes of 30 patients with unilateral APAC and 30 with NA were evaluated by ultrasound biomicroscopy (UBM) under light and dark conditions. UBM parameters such as anterior chamber depth (ACD), angle opening distance at 250 µm/500 µm from the scleral spur (AOD250/AOD500), trabecular ciliary process distance (TCPD) and iris-lens contact distance (ILCD) were measured in the superior (SQ) and inferior (IQ) quadrants. RESULTS: Significant differences between APAC fellow and NA eyes were found in ACD, P<0.001; AOD250 at SQ and IQ, P<0.001; AOD500 at SQ and IQ, P<0.001; TCPD light, P=0.010 and TCPD dark at SQ, P=0.031; and TCPD light at IQ, P=0.010. Significant differences between light and dark examinations of APAC fellow eyes were found in ILCD (P=0.009) at SQ and ILCD at IQ (P=0.006), and of NA eyes in ILCD at SQ (P=0.047) and ILCD at IQ (P<0.001). CONCLUSIONS: APAC fellow eyes have a more crowded anterior segment and shallower ACD than NA eyes. ILCD decreases in both groups when the illumination conditions change from light to dark.
Keywords: Eye; Microscopy; Ultrasonography; Biometry; Anterior chamber; Anterior chamber; Anterior eye segment; Anterior eye segment
RESUMO
OBJETIVO: Comparar características morfométricas entre olhos contralaterais com fechamento angular primário agudo (FAPA) e olhos glaucomatosos ou suspeitos com ângulo estreito (AE). MÉTODOS: Olhos contralaterais de 30 pacientes com FAPA unilateral e olhos de 30 pacientes com AE foram avaliados através da biomicroscopia ultra-sônica (BUS) no claro e escuro. Parâmetros da BUS como a profundidade central de câmara anterior (PCA), distância da abertura angular a 250 µm/500 µm do esporão escleral (AOD250/AOD500), distância entre o processo ciliar e o trabeculado (TCPD) e distância do contato iris-cristalino (ILCD) foram medidos nos quadrantes superior (QS) e inferior (QI). RESULTADOS: Diferenças significativas entre olhos contralaterais de FAPA e olhos com AE foram encontradas na PCA, p<0,001; AOD250 no QS e QI, p<0,001; AOD500 no QS e QI, p<0,001; TCPD no claro, p=0,010 e TCPD no escuro no QS, p=0,031; e TCPD no claro no QI, p=0,010. Diferenças significativas entre exames no claro e escuro realizados em olhos contralaterais com FAPA foram encontradas na ILCD (p=0,009) no QS e ILCD no QI (p=0,006), e em olhos com SE na ILCD no QS (p=0,047) e ILCD no QI (p<0,001). CONCLUSÕES: Olhos contralaterais de FAPA apresentam um segmento anterior mais aglomerado e uma PCA menor que olhos com AE. ILCD diminui em ambos os grupos quando as condições de iluminação mudam do claro para o escuro.
Descritores: Olho; Microscopia; Ultra-sonografia; Biometria; Câmara anterior; Câmara anterior; Segmento anterior do olho; Segmento anterior do olho
ORIGINAL ARTICLE
New comparative ultrasound biomicroscopic findings between fellow eyes of acute angle closure and glaucomatous eyes with narrow angle
Novos achados comparativos de biomicroscopia ultra-sônica entre olhos contralaterais com fechamento angular agudo e olhos glaucomatosos com ângulo estreito
Rafael Vidal MérulaI; Sebastião CronembergerII; Alberto Diniz FilhoI; Nassim CalixtoII
IPós-graduando, nível doutorado, da Faculdade de Medicina da Universidade Federal de Minas Gerais - UFMG - Belo Horizonte (MG) - Brasil
IILivre-docente, Professor Titular da Disciplina de Oftalmologia da Faculdade de Medicina da UFMG - Belo Horizonte (MG) - Brasil
ABSTRACT
PURPOSE: To compare morphometric features between fellow acute primary angle-closure (APAC) eyes and glaucomatous or suspect eyes with narrow angle (NA).
METHODS: Fellow eyes of 30 patients with unilateral APAC and 30 with NA were evaluated by ultrasound biomicroscopy (UBM) under light and dark conditions. UBM parameters such as anterior chamber depth (ACD), angle opening distance at 250 µm/500 µm from the scleral spur (AOD250/AOD500), trabecular ciliary process distance (TCPD) and iris-lens contact distance (ILCD) were measured in the superior (SQ) and inferior (IQ) quadrants.
RESULTS: Significant differences between APAC fellow and NA eyes were found in ACD, P<0.001; AOD250 at SQ and IQ, P<0.001; AOD500 at SQ and IQ, P<0.001; TCPD light, P=0.010 and TCPD dark at SQ, P=0.031; and TCPD light at IQ, P=0.010. Significant differences between light and dark examinations of APAC fellow eyes were found in ILCD (P=0.009) at SQ and ILCD at IQ (P=0.006), and of NA eyes in ILCD at SQ (P=0.047) and ILCD at IQ (P<0.001).
CONCLUSIONS: APAC fellow eyes have a more crowded anterior segment and shallower ACD than NA eyes. ILCD decreases in both groups when the illumination conditions change from light to dark.
Keywords: Eye/anatomy & histology; Microscopy/methods; Ultrasonography/methods; Biometry; Anterior chamber/anatomy & histology; Anterior chamber/ultrasonography; Anterior eye segment/anatomy & histology; Anterior eye segment/ultrasonography
RESUMO
OBJETIVO: Comparar características morfométricas entre olhos contralaterais com fechamento angular primário agudo (FAPA) e olhos glaucomatosos ou suspeitos com ângulo estreito (AE).
MÉTODOS: Olhos contralaterais de 30 pacientes com FAPA unilateral e olhos de 30 pacientes com AE foram avaliados através da biomicroscopia ultra-sônica (BUS) no claro e escuro. Parâmetros da BUS como a profundidade central de câmara anterior (PCA), distância da abertura angular a 250 µm/500 µm do esporão escleral (AOD250/AOD500), distância entre o processo ciliar e o trabeculado (TCPD) e distância do contato iris-cristalino (ILCD) foram medidos nos quadrantes superior (QS) e inferior (QI).
RESULTADOS: Diferenças significativas entre olhos contralaterais de FAPA e olhos com AE foram encontradas na
PCA, p<0,001; AOD250 no QS e QI, p<0,001; AOD500 no QS e QI, p<0,001; TCPD no claro, p=0,010 e TCPD no escuro no QS, p=0,031; e TCPD no claro no QI, p=0,010. Diferenças significativas entre exames no claro e escuro realizados em olhos contralaterais com FAPA foram encontradas na ILCD (p=0,009) no QS e ILCD no QI (p=0,006), e em olhos com SE na ILCD no QS (p=0,047) e ILCD no QI (p<0,001).
CONCLUSÕES: Olhos contralaterais de FAPA apresentam um segmento anterior mais aglomerado e uma PCA menor que olhos com AE. ILCD diminui em ambos os grupos quando as condições de iluminação mudam do claro para o escuro.
Descritores: Olho/anatomia & histologia; Microscopia/métodos; Ultra-sonografia/métodos; Biometria; Câmara anterior/anatomia & histologia; Câmara anterior/ultra-sonografia; Segmento anterior do olho/anatomia & histologia; Segmento anterior do olho/ultra-sonografia
INTRODUCTION
The most common mechanism in the development of acute primary angle closure (APAC) is an increased resistance caused by relative pupillary block, preventing the aqueous from flowing through the pupil(1-2). A laser peripheral iridotomy (LPI) would relieve this block and open the angle. Eyes with angle closure have important biometric differences from healthy eyes, such as a shallower anterior chamber, a thicker lens, and a shorter axial length(3). Ultrasound biomicroscopy (UBM) permits noninvasive examination of the anterior segment at high resolution (40 µm)(4). The efficacy of UBM has been demonstrated in relation to the mechanism of angle closure in eyes with primary angle closure glaucoma (PACG)(5), and the morphologic changes after laser iridotomy(6).
The aim of this paper was to perform UBM comparison among eyes that have an anatomic feature in common (narrow angle), eyes with the diagnosis or suspect characteristics of primary open-angle glaucoma (POAG) with narrow angle (NA) and fellow eyes of APAC patients.
METHODS
This was a prospective comparative observational case series. Patients with APAC and NA were consecutively recruited from the Glaucoma Service of São Geraldo Hospital/Federal University of Minas Gerais from September 2005 to June 2007. The study was carried out after approval by the Federal University of Minas Gerais Research Ethics Committee. Informed consent was obtained from all the participants. The following criteria were used to define APAC cases: 1) presence of at least two of the following symptoms: ocular or periocular pain, nausea and/or vomiting, antecedent history of intermittent blurring of vision with halos; and 2) presenting intraocular pressure (IOP) of more than 28 mmHg (Goldmann applanation tonometry) and the presence of at least three of the following signs: conjunctival injection, corneal epithelial edema, mid-dilated no reactive pupil, and shallow anterior chamber; and 3) the presence of an occluded angle in the affected eye (gonioscopy). The enrollment criteria for the fellow group were: 1) contralateral eyes of patients who had developed APAC; 2) narrow angle; 3) no previous ocular surgery or laser therapy; 4) no medications in use that affect pupillary reactions or diameter; 5) no abnormalities in cornea and iris; 6) no signs of secondary angle closure glaucoma; 7) no signs of plateau iris configuration (PIC) (the diagnosis of PIC requires normal central anterior chamber depth (ACD), peripheral iris anteriorly and centrally angled, and a flat or slightly convex iris by biomicroscopy, narrow angle under gonioscopic examination and the sign of double hump with the indentation gonioscopy. To confirm the diagnosis of PIC, the UBM examination shows the ciliary processes anteriorly located, closing the ciliary sulcus, and providing structural support behind the peripheral iris; 8) IOP that did not exceed 20 mmHg; and 9) no nuclear sclerosis defined as Lens Opacities Classification System (LOCS) II less than grade 2 (NC2, NO2)(7). Once the acute attack was broken, the fellow eye was evaluated clinically and by UBM. The group of NA was composed of patients with the diagnosis or suspect characteristics of POAG with NA. The criteria used to define POAG with NA were: 1) IOP higher than 21 mmHg; and 2) presence of glaucomatous optic neuropathy defined by at least two of the following items, cup/disc ratio (C/D) asymmetry between fellow eyes greater than 0.2, rim thinning, notching, C/D equal or more than 0.7, optic disc haemorrhage or retinal nerve fiber layer defect; and 3) glaucomatous visual field defects by automated perimetry (Octopus 1-2-3). The visual field was considered abnormal if two of the following three criteria were met on at least two consecutive examinations: (a) an abnormal Bebbie curve (deviation more than 0.0); (b) 3 contiguous nonedge points (allowing the two nasal step edge points) on a Octopus program G1 visual field with P<0.05 on the probability plot, with at least 1 point at P<0.01; and (c) a corrected loss variance P<0.05); and 4) narrow angle on gonioscopy. The criteria used to define eyes with suspect characteristics of POAG with NA were: the presence of the item 4, and the presence of aforementioned item 1 or 2 to define POAG with NA; however, the definition of item 2 was modified here (presence of one of the characteristics described above to define glaucomatous optic neuropathy). Exclusion criteria for NA were: nuclear sclerosis defined as LOCS II more than grade 2 (NC2, NO2) in both eyes; secondary glaucoma; PIC; signs of previous glaucoma crisis; corneal opacity in both eyes; necessity of surgical procedure to control IOP; previous surgical procedure in both eyes; and closed angle under dark gonioscopic examination. If one or more than one of the exclusion criteria for NA were identified in one eye, the other eye was evaluated clinically and by UBM before beginning the medication. If no exclusion criteria were found, the right eye was preferably evaluated. Clinical examination consisted of history, visual acuity with and without optical correction, biomicroscopy, gonioscopy and C/D examination. Gonioscopy was done by one of the authors (RVM) and confirmed by another investigator (SC), using a Goldmann 3 mirror goniolens (Volk Optical Inc, Mentor, Ohio, USA), and afterwards, a Zeiss 4-mirror goniolens (Carl Zeiss Meditec AG, Oberkochen, Germany) for indentation gonioscopy, in the undilated state under room light illumination (approximately 240 lux), and dark (approximately 0.1 lux) (the room was darkened in 5 minutes), both conditions previously measured with an illuminance meter [Minolta T10 Illuminance Meter, Konica Minolta Sensing Inc., Osaka, Japan]), the conditions being uniform for the whole examination. The gonioscopy classification currently used by the Glaucoma Service, both in primary gaze and under indentation, was adopted for this study and applied under room light illumination: 1) wide open angle (angle totally open); 2) intermediate open angle (when it was possible to see until scleral spur, but it was not possible to identify the ciliary band); 3) narrow open angle (when it was only possible to identify the anterior part of the trabecular meshwork); 4) closed angle (it was not possible to identify any structure). The angle was fully gonioscopically evaluated (360°), and the widest quadrant was adopted to label the angle of each eye.
UBM examination was performed with the commercial instrument (UBM model 840, Zeiss-Humphrey Instruments Inc., San Leandro, California,USA) and a 50-MHz transducer. After surface anesthesia with 0.5% proparacaine, a plastic eyecup containing 2% methylcellulose and physiologic saline was applied to the eyeball between the eyelids. The scanning was performed by placing the probe at the limbus and ciliary body region, always perpendicular to the surface of the eyeball, and multiple profile images of the limbic area in the superior (12 o'clock) and inferior (6 o'clock) meridians of the angle were obtained per eye with the patient in a supine position. An image centered on the pupil was also captured. The UBM examination was done in the undilated state under room light illumination (approximately 240 lux), and dark (approximately 0.1 lux), the conditions being uniform for the whole examination. Three clear and representative images per quadrant of the angle of each eye were selected for further analysis. The various parameters listed below were measured by a single observer (RVM) to rule out interobserver variability(8) on the images of the superior and inferior quadrants (SQ and IQ) using previously published methodology(9) (Figure 1).
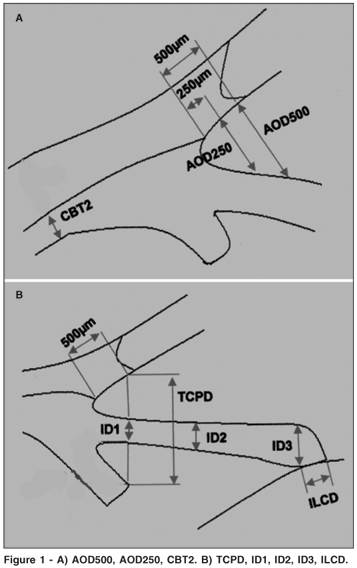
1. The central ACD, measured on a line extending from the corneal endothelium to the anterior lens surface perpendicularly to both mentioned anatomical structures.
2. The angle opening distance (AOD250) is the distance between the posterior corneal surface and the anterior iris surface measured on a line perpendicular to the trabecular meshwork, 250 µm from the scleral spur (Figure 1A).
3. The angle opening distance (AOD500) is the same as AOD250, however, 500 µm from the scleral spur (Figure 1A).
4. The trabecular ciliary process distance (TCPD), measured on a line extending from the corneal endothelium at 500 µm from the scleral spur perpendicularly through the iris to the ciliary processes (Figure 1B).
5. The iris thickness 1 (ID1), the iris thickness measured along the same line as the TCPD (Figure 1B).
6. Iris thickness 2 (ID2), the iris thickness 2 mm from the iris root (Figure 1B).
7. Iris thickness 3 (ID3), the maximum iris thickness near the pupillary edge (Figure 1B).
8. Ciliary body thickness 2 (CBT2), the ciliary body thickness measured at 2 mm from scleral spur (Figure 1A).
9. The iris-lens contact distance (ILCD), measured along the iris pigmented epithelium from the pupillary border to the point where the anterior lens surface leaves the iris (Figure 1B).
Statistical analyses were performed using 13.0 SPSS. We have used frequency histograms and the following tests: one-sample Kolmogorov-Smirnov, Student's t, paired Student's t, Mann-Whitney U, Wilcoxon and Pearson's chi-square. A P value of less than 0.05 was considered statistically significant.
RESULTS
Table 1 shows the demographic features of the 60 studied subjects. We assessed 30 fellow APAC eyes and 30 NA eyes. Twenty-one (70%) patients of the NA group had the diagnosis of POAG with narrow angle and 9 (30%) suspect characteristics. NA patients were older than APAC patients (P=0.003), however, no difference was found between the two groups when gender (P=0.559), race (P=0.435) and history of glaucoma (P=0.519) were compared. The statistical difference of gonioscopic classification was evident between groups (P<0.001).
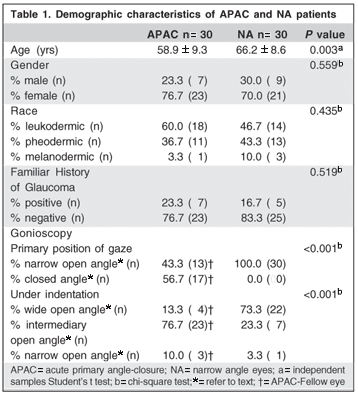
Table 2 shows the UBM parameters in APAC fellow eyes and NA eyes. Significant differences between APAC fellow and NA eyes were found in the ACD light (P<0.001), ACD dark (P<0.001), AOD250 at SQ light (P<0.001), AOD250 at SQ dark (P=0.001), AOD500 at SQ light (P<0.001), AOD500 at SQ dark (P<0.001), TCPD at SQ light (P=0.010), TCPD at SQ dark (P=0.031), ID3 at SQ dark (P=0.035), AOD250 at IQ light (P<0.001), AOD250 at IQ dark (P<0.001), AOD500 at IQ light (P<0.001), AOD500 at IQ dark (P<0.001) and TCPD at IQ light (P=0.010).
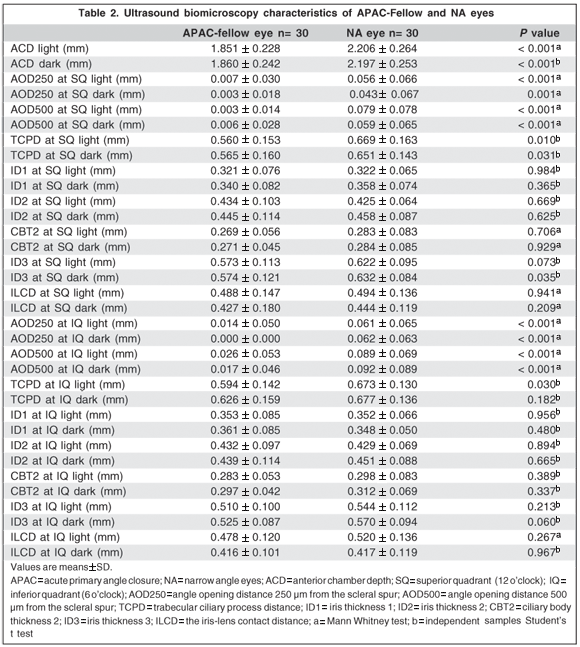
Table 3 shows the comparison between light and dark UBM measurements of APAC fellow and NA eyes. Significant differences between light and dark examinations of APAC fellow eyes were found in the ILCD at SQ (P=0.009) and ILCD at IQ (P=0.006); and between light and dark measurements of NA eyes were found in ID1 at SQ (P=0.008), ID2 at SQ (P=0.017), ILCD at SQ (P=0.047) and ILCD at IQ (P<0.001).
DISCUSSION
To the best of our knowledge, this is the first study that compared eleven UBM measurements between APAC fellow and NA eyes. Except for the age, both groups had similar demographic characteristics. The APAC patients had a mean age of 58.9 ± 9.3 years which was lower than that of the NA patients (66.2 ± 8.6 years) (P=0.003). This difference could be considered a limitation of this study owing to the fact that some changes in the structure of the eye occur when people get old(10). However, the differences in the anatomical features between the 6th and 7th decades, which are the decades of the majority of patients included in this study, are not so significant(10-11).
It has already been reported that fellow eyes of acute PACG have different topologic features and a higher incidence of appositional angle closure than normotensive narrow-angled eyes(12). Our UBM data demonstrated quite clearly that APAC fellow eyes are characterized by a more crowded anterior segment and a shallower ACD compared to NA eyes (Table 2). A shallower ACD in eyes with angle closure has already been reported previously, not only in biometric studies that compared these eyes with normal subjects(13-14), or with narrow angles(15), or with occludable angles(16), or with POAG(17), but also in UBM studies that compared angle closure eyes with normal subjects(18-19). Subgroups of studied primary angle closure glaucoma using UBM(18) presented a shallower ACD in the acute/intermittent form when compared to the chronic form.
The TCPD is a parameter of primary importance since it indicates the gap available for the iris between the trabecular meshwork and the ciliary process(9). In this series, TCPD values were higher in NA eyes compared to APAC fellow eyes, in light or dark conditions (Table 2). However, no significant difference was detected regarding ID1 (Table 2). These differences in TCPD have an effect on the AOD250 and AOD500, which were particularly short in APAC eyes (Table 2). Some studies evaluated the difference of TCPD between PACG and normal eyes in the South Indian population, but in subjects with PACG after LPI(19), between acute/intermittent PACG and chronic PACG eyes(18), and between primary angle closure suspects (PACS) and normal eyes(20), and the lower values were found in PACG, acute/intermittent PACG eyes and PACS, respectively. Some studies verified that contralateral eyes of acute angle closure and PACS eyes had lower values of AOD500 compared to healthy controls(20-21). Marchini et al.(18) showed statistically significant difference in AOD500 between acute/intermittent and chronic PACG eyes. The presence of a difference in TCPD in APAC fellow eyes compared to NA eyes could give an indication about the pathogenesis of the acute disease in the absence of a considerable opalescent lens.
For the first time, the iris thickness comparison between APAC fellow and NA eyes is being reported. The thickness of the iris (ID1, ID2 and ID3) was the same as that in APAC fellow eyes and in NA eyes, except for ID3 at SQ in dark conditions that showed marginal significance (P=0.035) with minor difference which could be an incidental situation. Probably, the iris thickness was not responsible for the narrowing of the angle. No difference in the ID1 between patients with PACG and normal subjects was found in one study(18) however it was noticed in another study with marginal difference(19). The finding of a thicker iris has been reported in Eskimos(22). In our study, unexpected findings were verified when comparison between light and dark examinations of iris thickness in NA group was performed; ID1 at SQ (P=0.008) and ID2 at SQ (P=0.017) were thicker in dark conditions (Table 3); these results were not evident in APAC fellow eyes. Regarding the CBT2, our data did not show any statistically significant difference either between the two groups or within the groups between light and dark conditions. Gohdo et al.(23), found a thinner ciliary body in narrow angle eyes in comparison to normal control eyes, and suggested that this thinning could be an age-related change.
The APAC fellow group in comparison to the NA group showed no statistically significant difference in the ILCD. However, when this parameter was evaluated between light and dark circumstances it had higher values in light conditions (Table 3). Probably, when mid-dilation occurs resulting in a pupillary block (simulated by the dark examination), the iris convexity increases according to a shallower ACD and older age(24), and, subsequently, ILCD appears to decrease. In our study, no difference was found in the iris thickness of the APAC group between light and dark conditions (Table 3).
In conclusion, this study demonstrates that APAC fellow eyes have a more crowded anterior segment, especially the entrance of the angle, as well as the decrease of ILCD when the illumination conditions change from light to dark. Longitudinal comparisons are required to further understand the differences in pathology of angle closures.
REFERENCES
1. Barkan O. Glaucoma: classification, causes, and surgical control. Results of microgonioscopic research. Am J Ophthalmol. 1938;21:1099-117.
2. Sugar HS. Newer concepts in classification of glaucomas. Am J Ophthalmol. 1949;32(3):425-33.
3. Lowe RF. Primary angle closure glaucoma: a review of ocular biometry. Aust NZ J Ophthalmol. 1977;5:9-17.
4. Pavlin CJ, Sherar MD, Foster FS. Subsurface ultrasound microscopic imaging of the intact eye. Ophthalmology. 1990;97(2):244-50.
5. Ritch R, Liebmann JM. Role of ultrasound biomicroscopy in the differentiation of block glaucomas. Curr Opin Ophthalmol. 1998;9(2):39-45.
6. Gazzard G, Friedman DS, Devereux JG, Chew P, Seah SK. A prospective ultrasound biomicroscopy evaluation of changes in anterior segment morphology after laser iridotomy in Asian eyes. Ophthalmology. 2003;110(3):630-8.
7. Chylack LT Jr, Leske MC, McCarthy D, Khu P, Kashinagi T, Sperduto R. Lens opacities classification system II (LOCS II). Arch Ophtahlmol. 1989;107(7):991-7.
8. Tello C, Liebmann J, Potash SD, Cohen H, Ritch R. Measurement of ultrasound biomicroscopy images: intraobserver and interobserver reliability. Invest Ophthalmol Vis Sci. 1994;35(9):3549-52.
9. Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992; 113(4):381-9.
10. Lim KJ, Hyung SM, Youn DH. Ocular dimensions with aging in normal eyes. Korean J Ophthalmol. 1992;6(1):19-31.
11. Markowitz SN, Morin JD. Angle closure glaucoma: relation between lens thickness, anterior chamber depth and age. Can J Ophthalmol. 1984;19(7):300-2.
12. Sawada A, Sakuma T, Yamamoto T, Kitazawa Y. Appositional angle closure in eyes with narrow angles: comparison between the fellow eyes of acute angle closure glaucoma and normotensive cases. J Glaucoma. 1997;6(5):288-92.
13. Saxena S, Agrawal PK, Pratap VB, Nath R. Anterior chamber depth and lens thickness in primary angle closure glaucoma: a case-control study. Indian J Ophthalmol. 1993;41(2):71-3.
14. Sihota R, Gupta V, Agarwal HC, Pandey RM, Deepak KK. Comparison of symptomatic and asymptomatic, chronic, primary angle closure glaucoma, open-angle glaucoma, and controls. J Glaucoma. 2000;9(3):208-13.
15. Lee DA, Brubaker RF, Illstrup DM. Anterior chamber dimensions in patients with narrow angles and angle closure glaucoma. Arch Ophthalmol. 1984; 102(1):46-50.
16. George R, Paul PG, Baskaran M, Ramesh SV, Raju P, Arvind H, et al. Ocular biometry in occludable angles and angle closure glaucoma: a population based survey. Br J Ophthalmol. 2003;87(4):399-402.
17. Calixto N, Cronemberger S. Glaucoma simples x glaucoma agudo: estudo eco-biométrico. Arq Bras Oftalmol. 1986;49(1):1-8.
18. Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle closure glaucoma. Ophthalmology. 1998;105(11):2091-8.
19. Garudadri CS, Chelerkar V, Nutheti R. An ultrasound biomicroscopic study of the anterior segment in Indian eyes with primary angle closure glaucoma. J Glaucoma. 2002;11(6):502-7.
20. Ramani KK, Mani B, Ronnie G, Joseph R, Lingam V. Gender variation in ocular biometry and ultrasound biomicroscopy of primary angle closure suspects and normal eyes. J Glaucoma. 2007;16(1):122-8.
21. Friedman DS, Gazzard G, Foster P, Devereux J, Broman A, Quigley H, et al. Ultrasonographic biomicroscopy, Scheimpflug photography, and novel provocative tests in contralateral eyes of Chinese patients initially seen with acute angle closure. Arch Ophthalmol. 2003;121(5):633-42.
22. Alsbirk PH. Primary angle closure glaucoma. Oculometry, epidemiology, and genetics in a high risk population. Acta Ophthalmol Suppl. 1976;(127):5-31.
23. Gohdo T, Tsumura T, Iijima H, Kashiwagi K, Tsukahara S. Ultrasound biomicroscopic study of ciliary body thickness in eyes with narrow angles. Am J Ophthalmol. 2000;129(3):342-6.
24. Nonaka A, Iwawaki T, Kikuchi M, Fujihara M, Nishida A, Kurimoto Y. Quantitative evaluation of iris convexity in primary angle closure. Am J Ophthalmol. 2007;143(4):695-7.
 Endereço para correspondência:
Endereço para correspondência:
Rafael Vidal Mérula
Rua Espírito Santo, 1.315 - Apto. 402
Juiz de Fora (MG) CEP 36016-200
E-mail: [email protected]
Recebido para publicação em 28.07.2008
Aprovação em 29.10.2008
Trabalho realizado no Serviço de Glaucoma do Hospital São Geraldo (Hospital das Clínicas) da Faculdade de Medicina da Universidade Federal de Minas Gerais - UFMG - Belo Horizonte (MG) - Brasil.